Hypoxic stress and cancer: imaging the axis of evil in tumor metastasis
- PMID: 21793071
- PMCID: PMC3558740
- DOI: 10.1002/nbm.1632
Hypoxic stress and cancer: imaging the axis of evil in tumor metastasis
Abstract
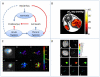
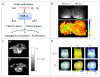
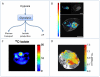
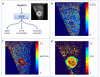
Tumors emerge as a result of the sequential acquisition of genetic, epigenetic and somatic alterations promoting cell proliferation and survival. The maintenance and expansion of tumor cells rely on their ability to adapt to changes in their microenvironment, together with the acquisition of the ability to remodel their surroundings. Tumor cells interact with two types of interconnected microenvironments: the metabolic cell autonomous microenvironment and the nonautonomous cellular-molecular microenvironment comprising interactions between tumor cells and the surrounding stroma. Hypoxia is a central player in cancer progression, affecting not only tumor cell autonomous functions, such as cell division and invasion, resistance to therapy and genetic instability, but also nonautonomous processes, such as angiogenesis, lymphangiogenesis and inflammation, all contributing to metastasis. Closely related microenvironmental stressors affecting cancer progression include, in addition to hypoxia, elevated interstitial pressure and oxidative stress. Noninvasive imaging offers multiple means to monitor the tumor microenvironment and its consequences, and can thus assist in the understanding of the biological basis of hypoxia and microenvironmental stress in cancer progression, and in the development of strategies to monitor therapies targeted at stress-induced tumor progression.
Copyright © 2011 John Wiley & Sons, Ltd.
Figures
References
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. - PubMed
-
- Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269–270. - PubMed
-
- Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. - PubMed
-
- Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134(5):703–707. - PubMed
-
- Milosevic M, Fyles A, Hedley D, Hill R. The human tumor microenvironment: invasive (needle) measurement of oxygen and interstitial fluid pressure. Semin Radiat Oncol. 2004;14(3):249–258. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

