Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins
- PMID: 21793199
- PMCID: PMC3214619
- DOI: 10.1002/ieam.258
Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins
Abstract
The primary aim of this article is to provide an overview of perfluoroalkyl and polyfluoroalkyl substances (PFASs) detected in the environment, wildlife, and humans, and recommend clear, specific, and descriptive terminology, names, and acronyms for PFASs. The overarching objective is to unify and harmonize communication on PFASs by offering terminology for use by the global scientific, regulatory, and industrial communities. A particular emphasis is placed on long-chain perfluoroalkyl acids, substances related to the long-chain perfluoroalkyl acids, and substances intended as alternatives to the use of the long-chain perfluoroalkyl acids or their precursors. First, we define PFASs, classify them into various families, and recommend a pragmatic set of common names and acronyms for both the families and their individual members. Terminology related to fluorinated polymers is an important aspect of our classification. Second, we provide a brief description of the 2 main production processes, electrochemical fluorination and telomerization, used for introducing perfluoroalkyl moieties into organic compounds, and we specify the types of byproducts (isomers and homologues) likely to arise in these processes. Third, we show how the principal families of PFASs are interrelated as industrial, environmental, or metabolic precursors or transformation products of one another. We pay particular attention to those PFASs that have the potential to be converted, by abiotic or biotic environmental processes or by human metabolism, into long-chain perfluoroalkyl carboxylic or sulfonic acids, which are currently the focus of regulatory action. The Supplemental Data lists 42 families and subfamilies of PFASs and 268 selected individual compounds, providing recommended names and acronyms, and structural formulas, as well as Chemical Abstracts Service registry numbers.
Copyright © 2011 SETAC.
Figures








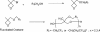
References
-
- [3M] Company. Fluorochemical use, distribution and release overview. USEPA Administrative Record AR226-0550. 1999. Available from: http://www.regulations.gov as document EPA-HQ-OPPT-2002-0051-0003.
-
- [3M] Company. Voluntary Use and Exposure Information Profile for Perfluorooctanoic Acid and Salts. USEPA Administrative Record AR226-0595. 2000a. Available from: http://www.regulations.gov as document EPA-HQ-OPPT-2002-0051-0009.
-
- [3M] Company. Re: CAS Names for PFOS-Based Products. USEPA Administrative Record AR226-0931. 2000b. Available from: http://OPPT.NCIC@epamail.epa.gov.
-
- [3M] Company. Re: Phase-Out Plan for POSF-based Products. USEPA Administrative Record AR226-0600. 2000c. Available from: http://www.regulations.gov, as document EPA-HQ-OPPT-2002-0051-0006.
-
- Ahrens L. Polyfluoroalkyl compounds in the aquatic environment: a review of their occurrence and fate. J Environ Monit. 2011;13:20–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

