Plasmonic-based imaging of local square wave voltammetry
- PMID: 21793508
- PMCID: PMC3288114
- DOI: 10.1021/ac201392r
Plasmonic-based imaging of local square wave voltammetry
Abstract
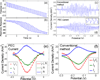
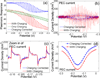
Square wave voltammetry (SWV) is widely used in electrochemical analysis and sensors because of its high sensitivity and efficient rejection of background current, but SWV by the conventional electrochemical detection method does not provide spatial resolution. We report here a plasmonic method to image local SWV, which opens the door for analyzing heterogeneous electrochemical reactions and for high-throughput detections of microarrays. We describe the basic principle, validate the principle by comparing the plasmonic-based SWV with those obtained with the conventional method, and demonstrate imaging capability for local electrochemical analysis.
Figures


Similar articles
-
Plasmonic Imaging of Electrochemical Reactions of Single Nanoparticles.Acc Chem Res. 2016 Nov 15;49(11):2614-2624. doi: 10.1021/acs.accounts.6b00348. Epub 2016 Sep 23. Acc Chem Res. 2016. PMID: 27662069
-
Imaging local electrochemical current via surface plasmon resonance.Science. 2010 Mar 12;327(5971):1363-6. doi: 10.1126/science.1186476. Science. 2010. PMID: 20223983
-
Microscale, Electrochemical, Aptamer-Based Sensors for Enhanced Small-Molecule Detection at Millisecond Time Scales.ACS Sens. 2023 Dec 22;8(12):4521-4530. doi: 10.1021/acssensors.3c01055. Epub 2023 Nov 21. ACS Sens. 2023. PMID: 38104257 Free PMC article.
-
Electrochemical Sensors for Determination of Bromate in Water and Food Samples-Review.Biosensors (Basel). 2021 May 27;11(6):172. doi: 10.3390/bios11060172. Biosensors (Basel). 2021. PMID: 34072226 Free PMC article. Review.
-
Optical methods for studying local electrochemical reactions with spatial resolution: A critical review.Anal Chim Acta. 2019 Oct 3;1074:1-15. doi: 10.1016/j.aca.2019.02.053. Epub 2019 Mar 7. Anal Chim Acta. 2019. PMID: 31159929 Review.
Cited by
-
Plasmonic-based electrochemical impedance spectroscopy: application to molecular binding.Anal Chem. 2012 Jan 3;84(1):327-33. doi: 10.1021/ac202634h. Epub 2011 Dec 14. Anal Chem. 2012. PMID: 22122514 Free PMC article.
-
High-resolution impedance mapping using electrically activated quantitative phase imaging.Light Sci Appl. 2021 Jan 21;10(1):20. doi: 10.1038/s41377-020-00461-x. Light Sci Appl. 2021. PMID: 33479199 Free PMC article.
-
Imaging the electrocatalytic activity of single nanoparticles.Nat Nanotechnol. 2012 Oct;7(10):668-72. doi: 10.1038/nnano.2012.134. Epub 2012 Aug 26. Nat Nanotechnol. 2012. PMID: 22922540
-
Sensitive Imaging of Electroactive Species in Plasmonic Electrochemical Microscopy Enabled by Nanoconfinement.ACS Electrochem. 2025 Feb 17;1(6):974-986. doi: 10.1021/acselectrochem.4c00227. eCollection 2025 Jun 5. ACS Electrochem. 2025. PMID: 40496335 Free PMC article.
References
-
- Fernandez JL, Walsh DA, Bard AJ. Thermodynamic guidelines for the design of bimetallic catalysts for oxygen electroreduction and rapid screening by scanning electrochemical microscopy. M-Co (M : Pd, Ag, Au) Journal of the American Chemical Society. 2005;127:357–365. - PubMed
-
- Sanchez-Sanchez CM, Solla-Gullon J, Vidal-Iglesias FJ, Aldaz A, Montiel V, Herrero E. Imaging Structure Sensitive Catalysis on Different Shape-Controlled Platinum Nanoparticles. Journal of the American Chemical Society. 2010;132 5622-+ - PubMed
-
- Mellander L, Cans AS, Ewing AG. Electrochemical Probes for Detection and Analysis of Exocytosis and Vesicles. ChemPhysChem. 2010;11:2756–2763. - PubMed
-
- Fojta M. Electrochemical sensors for DNA interactions and damage. Electroanalysis. 2002;14:1449–1463.
-
- Amemiya S, Bard AJ, Fan F-RF, Mirkin MV, Unwin PR. Scanning Electrochemical Microscopy. Annual Review of Analytical Chemistry. 2008;1:95–131. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

