Mechanisms controlling virulence thresholds of mixed viral populations
- PMID: 21795346
- PMCID: PMC3196390
- DOI: 10.1128/JVI.00355-11
Mechanisms controlling virulence thresholds of mixed viral populations
Abstract
The propensity of RNA viruses to revert attenuating mutations contributes to disease and complicates vaccine development. Despite the presence of virulent revertant viruses in some live-attenuated vaccines, disease from vaccination is rare. This suggests that in mixed viral populations, attenuated viruses may limit the pathogenesis of virulent viruses, thus establishing a virulence threshold. Here we examined virulence thresholds using mixtures of virulent and attenuated viruses in a transgenic mouse model of poliovirus infection. We determined that a 1,000-fold excess of the attenuated Sabin strain of poliovirus was protective against disease induced by the virulent Mahoney strain. Protection was induced locally, and inactivated virus conferred protection. Treatment with a poliovirus receptor-blocking antibody phenocopied the protective effect of inactivated viruses in vitro and in vivo, suggesting that one mechanism controlling virulence thresholds may be competition for a viral receptor. Additionally, the type I interferon response reduces poliovirus pathogenesis; therefore, we examined virulence thresholds in mice lacking the alpha/beta interferon receptor. We found that the attenuated virus was virulent in immunodeficient mice due to the enhanced replication and reversion of attenuating mutations. Therefore, while the type I interferon response limits the virulence of the attenuated strain by reducing replication, protection from disease conferred by the attenuated strain in immunocompetent mice can occur independently of replication. Our results identified mechanisms controlling the virulence of mixed viral populations and indicate that live-attenuated vaccines containing virulent virus may be safe, as long as virulent viruses are present at levels below a critical threshold.
Figures
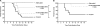


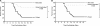


Similar articles
-
Characterization of formaldehyde-inactivated poliovirus preparations made from live-attenuated strains.J Gen Virol. 2003 Jul;84(Pt 7):1781-1788. doi: 10.1099/vir.0.19088-0. J Gen Virol. 2003. PMID: 12810872
-
Evolution of the Sabin type 1 poliovirus in humans: characterization of strains isolated from patients with vaccine-associated paralytic poliomyelitis.J Virol. 1997 Oct;71(10):7758-68. doi: 10.1128/JVI.71.10.7758-7768.1997. J Virol. 1997. PMID: 9311861 Free PMC article.
-
Rational design of genetically stable, live-attenuated poliovirus vaccines of all three serotypes: relevance to poliomyelitis eradication.J Virol. 2006 Sep;80(17):8653-63. doi: 10.1128/JVI.00370-06. J Virol. 2006. PMID: 16912313 Free PMC article.
-
One hundred years of poliovirus pathogenesis.Virology. 2006 Jan 5;344(1):9-16. doi: 10.1016/j.virol.2005.09.015. Virology. 2006. PMID: 16364730 Review.
-
Poliovirus attenuation and pathogenesis in a transgenic mouse model for poliomyelitis.Dev Biol Stand. 1993;78:109-16. Dev Biol Stand. 1993. PMID: 8388820 Review.
Cited by
-
Viral population dynamics and virulence thresholds.Curr Opin Microbiol. 2012 Aug;15(4):525-30. doi: 10.1016/j.mib.2012.05.007. Epub 2012 Jun 2. Curr Opin Microbiol. 2012. PMID: 22658738 Free PMC article. Review.
-
Co-detection of SARS-CoV-2 and other respiratory pathogens: Lessons from the field to face the second wave.J Clin Virol. 2020 Dec;133:104658. doi: 10.1016/j.jcv.2020.104658. Epub 2020 Oct 15. J Clin Virol. 2020. PMID: 33126110 Free PMC article. No abstract available.
-
Advancing Vaccine Strategies against Candida Infections: Exploring New Frontiers.Vaccines (Basel). 2023 Oct 29;11(11):1658. doi: 10.3390/vaccines11111658. Vaccines (Basel). 2023. PMID: 38005990 Free PMC article. Review.
-
Mutagenesis-mediated decrease of pathogenicity as a feature of the mutant spectrum of a viral population.PLoS One. 2012;7(6):e39941. doi: 10.1371/journal.pone.0039941. Epub 2012 Jun 28. PLoS One. 2012. PMID: 22761933 Free PMC article.
-
Vaccines against candidiasis: Status, challenges and emerging opportunity.Front Cell Infect Microbiol. 2022 Aug 18;12:1002406. doi: 10.3389/fcimb.2022.1002406. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36061876 Free PMC article. Review.
References
-
- . 2009. Update on vaccine-derived polioviruses—worldwide, January 2008-June 2009. MMWR Morb. Mortal. Wkly. Rep. 58:1002–1006 - PubMed
-
- Barton E. S., Connolly J. L., Forrest J. C., Chappell J. D., Dermody T. S. 2001. Utilization of sialic acid as a coreceptor enhances reovirus attachment by multistep adhesion strengthening. J. Biol. Chem. 276:2200–2211 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

