Phase I study of PARP inhibitor ABT-888 in combination with topotecan in adults with refractory solid tumors and lymphomas
- PMID: 21795476
- PMCID: PMC3166628
- DOI: 10.1158/0008-5472.CAN-11-1227
Phase I study of PARP inhibitor ABT-888 in combination with topotecan in adults with refractory solid tumors and lymphomas
Abstract
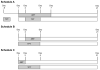
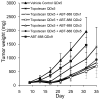
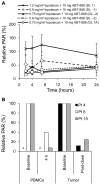
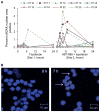
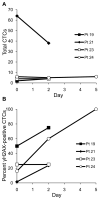
A phase I trial of ABT-888 (veliparib), a PARP inhibitor, in combination with topotecan, a topoisomerase I-targeted agent, was carried out to determine maximum tolerated dose (MTD), safety, pharmacokinetics, and pharmacodynamics of the combination in patients with refractory solid tumors and lymphomas. Varying schedules and doses of intravenous topotecan in combination with ABT-888 (10 mg) administered orally twice a day (BID) were evaluated. Plasma and urine pharmacokinetics were assessed and levels of poly(ADP-ribose) (PAR) and the DNA damage marker γH2AX were measured in tumor and peripheral blood mononuclear cells (PBMC). Twenty-four patients were enrolled. Significant myelosuppression limited the ability to coadminister ABT-888 with standard doses of topotecan, necessitating dose reductions. Preclinical studies using athymic mice carrying human tumor xenografts also informed schedule changes. The MTD was established as topotecan 0.6 mg/m²/d and ABT-888 10 mg BID on days one to five of 21-day cycles. Topotecan did not alter the pharmacokinetics of ABT-888. A more than 75% reduction in PAR levels was observed in 3 paired tumor biopsy samples; a greater than 50% reduction was observed in PBMCs from 19 of 23 patients with measurable levels. Increases in γH2AX response in circulating tumor cells (CTC) and PBMCs were observed in patients receiving ABT-888 with topotecan. We show a mechanistic interaction of a PARP inhibitor, ABT-888, with a topoisomerase I inhibitor, topotecan, in PBMCs, tumor, and CTCs. Results of this trial reveal that PARP inhibition can modulate the capacity to repair topoisomerase I-mediated DNA damage in the clinic.
Trial registration: ClinicalTrials.gov NCT00553189.
©2011 AACR.
Figures
References
-
- Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays. 2004;26:882–93. - PubMed
-
- Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–40. - PubMed
-
- Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. - PubMed
-
- Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–7. - PubMed
-
- Donawho CK, Luo Y, Penning TD, Bauch JL, Bouska JJ, Bontcheva-Diaz VD, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007;13:2728–37. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

