Longitudinal development of human brain wiring continues from childhood into adulthood
- PMID: 21795544
- PMCID: PMC6623097
- DOI: 10.1523/JNEUROSCI.5302-10.2011
Longitudinal development of human brain wiring continues from childhood into adulthood
Abstract
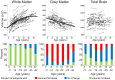
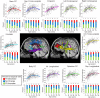
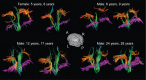
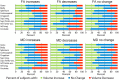
Healthy human brain development is a complex process that continues during childhood and adolescence, as demonstrated by many cross-sectional and several longitudinal studies. However, whether these changes end in adolescence is not clear. We examined longitudinal white matter maturation using diffusion tensor tractography in 103 healthy subjects aged 5-32 years; each volunteer was scanned at least twice, with 221 total scans. Fractional anisotropy (FA) and mean diffusivity (MD), parameters indicative of factors including myelination and axon density, were assessed in 10 major white matter tracts. All tracts showed significant nonlinear development trajectories for FA and MD. Significant within-subject changes occurred in the vast majority of children and early adolescents, and these changes were mostly complete by late adolescence for projection and commissural tracts. However, association tracts demonstrated postadolescent within-subject maturation of both FA and MD. Diffusion parameter changes were due primarily to decreasing perpendicular diffusivity, although increasing parallel diffusivity contributed to the prolonged increases of FA in association tracts. Volume increased significantly with age for most tracts, and longitudinal measures also demonstrated postadolescent volume increases in several association tracts. As volume increases were not directly associated with either elevated FA or reduced MD between scans, the observed diffusion parameter changes likely reflect microstructural maturation of brain white matter tracts rather than just gross anatomy.
Figures




References
-
- Ashtari M, Cervellione KL, Hasan KM, Wu J, McIlree C, Kester H, Ardekani BA, Roofeh D, Szeszko PR, Kumra S. White matter development during late adolescence in healthy males: a cross-sectional diffusion tensor imaging study. Neuroimage. 2007;35:501–510. - PubMed
-
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–1854. - PubMed
-
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15:435–455. - PubMed
-
- Ben Bashat D, Ben Sira L, Graif M, Pianka P, Hendler T, Cohen Y, Assaf Y. Normal white matter development from infancy to adulthood: comparing diffusion tensor and high b value diffusion weighted MR images. J Magn Reson Imaging. 2005;21:503–511. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
