Frequency-tuned cerebellar channels and burst-induced LTD lead to the cancellation of redundant sensory inputs
- PMID: 21795551
- PMCID: PMC6623080
- DOI: 10.1523/JNEUROSCI.0193-11.2011
Frequency-tuned cerebellar channels and burst-induced LTD lead to the cancellation of redundant sensory inputs
Abstract
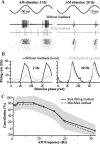
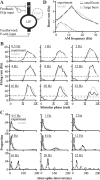
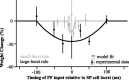
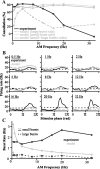
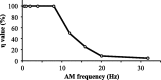
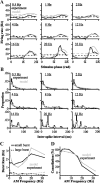
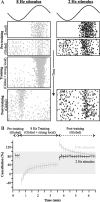
For optimal sensory processing, neural circuits must extract novel, unpredictable signals from the redundant sensory input in which they are embedded, but the detailed cellular and network mechanisms that implement such selective cancellation are presently unknown. Using a combination of modeling and experiment, we characterize in detail a cerebellar circuit in weakly electric fish, showing how it can carry out this computation. We use a model incorporating the wide range of experimentally estimated parallel fiber feedback delays and a burst-induced LTD rule derived from in vitro experiments to explain the precise cancellation of redundant signals observed in vivo. Our model demonstrates how the backpropagation-dependent burst dynamics adjusts the temporal pairing width of the plasticity mechanism to precisely match the frequency of the redundant signal. The model also makes the prediction that this cerebellar feedback pathway must be composed of frequency-tuned channels; this prediction is subsequently verified in vivo, highlighting a novel and general capability of cerebellar circuitry.
Figures
Similar articles
-
Burst-induced anti-Hebbian depression acts through short-term synaptic dynamics to cancel redundant sensory signals.J Neurosci. 2010 Apr 28;30(17):6152-69. doi: 10.1523/JNEUROSCI.0303-10.2010. J Neurosci. 2010. PMID: 20427673 Free PMC article.
-
Dendritic SK channels convert NMDA-R-dependent LTD to burst timing-dependent plasticity.J Neurophysiol. 2013 Dec;110(12):2689-703. doi: 10.1152/jn.00506.2013. Epub 2013 Sep 18. J Neurophysiol. 2013. PMID: 24047910
-
Learning contrast-invariant cancellation of redundant signals in neural systems.PLoS Comput Biol. 2013;9(9):e1003180. doi: 10.1371/journal.pcbi.1003180. Epub 2013 Sep 12. PLoS Comput Biol. 2013. PMID: 24068898 Free PMC article.
-
Systems biology perspectives on cerebellar long-term depression.Neurosignals. 2008;16(4):300-17. doi: 10.1159/000123040. Epub 2008 Jul 18. Neurosignals. 2008. PMID: 18635946 Review.
-
Neural mechanisms for filtering self-generated sensory signals in cerebellum-like circuits.Curr Opin Neurobiol. 2011 Aug;21(4):602-8. doi: 10.1016/j.conb.2011.05.031. Epub 2011 Jun 23. Curr Opin Neurobiol. 2011. PMID: 21704507 Review.
Cited by
-
Feedback optimizes neural coding and perception of natural stimuli.Elife. 2018 Oct 5;7:e38935. doi: 10.7554/eLife.38935. Elife. 2018. PMID: 30289387 Free PMC article.
-
Serotonin Selectively Increases Detectability of Motion Stimuli in the Electrosensory System.eNeuro. 2018 May 25;5(3):ENEURO.0013-18.2018. doi: 10.1523/ENEURO.0013-18.2018. eCollection 2018 May-Jun. eNeuro. 2018. PMID: 29845105 Free PMC article.
-
Internally Generated Predictions Enhance Neural and Behavioral Detection of Sensory Stimuli in an Electric Fish.Neuron. 2018 Jul 11;99(1):135-146.e3. doi: 10.1016/j.neuron.2018.06.006. Neuron. 2018. PMID: 30001507 Free PMC article.
-
Population Coding and Correlated Variability in Electrosensory Pathways.Front Integr Neurosci. 2018 Nov 27;12:56. doi: 10.3389/fnint.2018.00056. eCollection 2018. Front Integr Neurosci. 2018. PMID: 30542271 Free PMC article. Review.
-
Complexity Collapse, Fluctuating Synchrony, and Transient Chaos in Neural Networks With Delay Clusters.Front Syst Neurosci. 2021 Nov 19;15:720744. doi: 10.3389/fnsys.2021.720744. eCollection 2021. Front Syst Neurosci. 2021. PMID: 34867219 Free PMC article.
References
-
- Albus S. A theory of cerebellar function. Math Biosci. 1971;10:25–61.
-
- Bastian J, Nguyenkim J. Dendritic modulation of burst-like firing in sensory neurons. J Neurophysiol. 2001;85:10–22. - PubMed
-
- Bastian J, Chacron MJ, Maler L. Plastic and nonplastic pyramidal cells perform unique roles in a network capable of adaptive redundancy reduction. Neuron. 2004;41:767–779. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
