TCRs used in cancer gene therapy cross-react with MART-1/Melan-A tumor antigens via distinct mechanisms
- PMID: 21795600
- PMCID: PMC3166883
- DOI: 10.4049/jimmunol.1101268
TCRs used in cancer gene therapy cross-react with MART-1/Melan-A tumor antigens via distinct mechanisms
Abstract
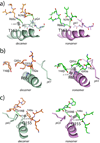
T cells engineered to express TCRs specific for tumor Ags can drive cancer regression. The first TCRs used in cancer gene therapy, DMF4 and DMF5, recognize two structurally distinct peptide epitopes of the melanoma-associated MART-1/Melan-A protein, both presented by the class I MHC protein HLA-A*0201. To help understand the mechanisms of TCR cross-reactivity and provide a foundation for the further development of immunotherapy, we determined the crystallographic structures of DMF4 and DMF5 in complex with both of the MART-1/Melan-A epitopes. The two TCRs use different mechanisms to accommodate the two ligands. Although DMF4 binds the two with a different orientation, altering its position over the peptide/MHC, DMF5 binds them both identically. The simpler mode of cross-reactivity by DMF5 is associated with higher affinity toward both ligands, consistent with the superior functional avidity of DMF5. More generally, the observation of two diverging mechanisms of cross-reactivity with the same Ags and the finding that TCR-binding orientation can be determined by peptide alone extend our understanding of the mechanisms underlying TCR cross-reactivity.
Figures



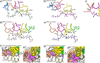
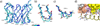

References
-
- Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich JR, Lee CC, Restifo NP, Schwarz SL, Cogdill AP, Bishop RJ, Kim H, Brewer CC, Rudy SF, VanWaes C, Davis JL, Mathur A, Ripley RT, Nathan DA, Laurencot CM, Rosenberg SA. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. - PMC - PubMed
-
- Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer Regression in Patients After Transfer of Genetically Engineered Lymphocytes. Science. 2006;314:126–129. - PMC - PubMed
-
- Johnson LA, Heemskerk B, Powell DJ, Jr, Cohen CJ, Morgan RA, Dudley ME, Robbins PF, Rosenberg SA. Gene Transfer of Tumor-Reactive TCR Confers Both High Avidity and Tumor Reactivity to Nonreactive Peripheral Blood Mononuclear Cells and Tumor-Infiltrating Lymphocytes. J Immunol. 2006;177:6548–6559. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

