Cholinergic modulation of working memory activity in primate prefrontal cortex
- PMID: 21795623
- PMCID: PMC3214100
- DOI: 10.1152/jn.00148.2011
Cholinergic modulation of working memory activity in primate prefrontal cortex
Abstract
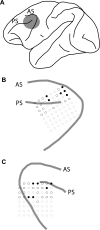
The prefrontal cortex, a cortical area essential for working memory and higher cognitive functions, is modulated by a number of neurotransmitter systems, including acetylcholine; however, the impact of cholinergic transmission on prefrontal activity is not well understood. We relied on systemic administration of a muscarinic receptor antagonist, scopolamine, to investigate the role of acetylcholine on primate prefrontal neuronal activity during execution of working memory tasks and recorded neuronal activity with chronic electrode arrays and single electrodes. Our results indicated a dose-dependent decrease in behavioral performance after scopolamine administration in all the working memory tasks we tested. The effect could not be accounted for by deficits in visual processing, eye movement responses, or attention, because the animals performed a visually guided saccade task virtually error free, and errors to distracting stimuli were not increased. Performance degradation under scopolamine was accompanied by decreased firing rate of the same cortical sites during the delay period of the task and decreased selectivity for the spatial location of the stimuli. These results demonstrate that muscarinic blockade impairs performance in working memory tasks and prefrontal activity mediating working memory.
Figures




References
-
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry 57: 1377–1384, 2005 - PubMed
-
- Barraclough DJ, Conroy ML, Lee D. Prefrontal cortex and decision making in a mixed-strategy game. Nat Neurosci 7: 404–410, 2004 - PubMed
-
- Bartus RT, Johnson HR. Short-term memory in the rhesus monkey: disruption from the anti-cholinergic scopolamine. Pharmacol Biochem Behav 5: 39–46, 1976 - PubMed
-
- Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science 217: 408–414, 1982 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

