Decreased hyperpolarization-activated currents in layer 5 pyramidal neurons enhances excitability in focal cortical dysplasia
- PMID: 21795624
- PMCID: PMC3214088
- DOI: 10.1152/jn.00164.2011
Decreased hyperpolarization-activated currents in layer 5 pyramidal neurons enhances excitability in focal cortical dysplasia
Abstract
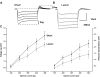
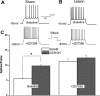
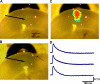
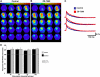
Focal cortical dysplasia is associated with the development of seizures in children and is present in up to 40% of intractable childhood epilepsies. Transcortical freeze lesions in newborn rats reproduce many of the anatomical and physiological characteristics of human cortical dysplasia. Rats with freeze lesions have increased seizure susceptibility and a region of hyperexcitable cortex adjacent to the lesion. Since alterations in hyperpolarization-activated nonspecific cation (HCN) channels are often associated with epilepsy, we used whole cell patch-clamp recording and voltage-sensitive dye imaging to examine alterations in HCN channels and inwardly rectifying hyperpolarization-activated currents (I(h)) in cortical dysplasia. (L5) pyramidal neurons in lesioned animals had hyperpolarized resting membrane potentials, increased input resistances and reduced voltage "sag" associated with I(h) activation. These differences became nonsignificant after application of the I(h) blocker ZD7288. Temporal excitatory postsynaptic potential (EPSP) summation and intrinsic excitability were increased in neurons near the freeze lesion. Using voltage-sensitive dye imaging of neocortical slices, we found that inhibiting I(h) with ZD7288 increased the half-width of dye signals. The anticonvulsant lamotrigine produced a significant decrease in spread of activity. The ability of lamotrigine to decrease network activity was reduced in the hyperexcitable cortex near the freeze lesion. These results suggest that I(h) serves to constrain network activity in addition to its role in regulating cellular excitability. Reduced I(h) may contribute to increased network excitability in cortical dysplasia.
Figures





References
-
- Avanzini G, Franceschetti S, Mantegazza M. Epileptogenic channelopathies: experimental models of human pathologies. Epilepsia 48: 51–64, 2007 - PubMed
-
- Bandyopadhyay S, Hablitz JJ. NR2B antagonists restrict spatiotemporal spread of activity in a rat model of cortical dysplasia. Epilepsy Res 72: 127–139, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

