The PGC-1α-related coactivator promotes mitochondrial and myogenic adaptations in C2C12 myotubes
- PMID: 21795630
- PMCID: PMC3197342
- DOI: 10.1152/ajpregu.00232.2011
The PGC-1α-related coactivator promotes mitochondrial and myogenic adaptations in C2C12 myotubes
Abstract
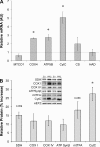
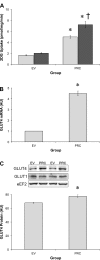
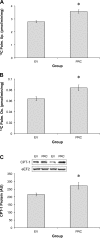
The transcriptional coactivator PGC-1α is a potent regulator of skeletal muscle metabolism. Less is known about the structurally similar PGC-1α-related coactivator (PRC) that is enriched in myoblasts and adult skeletal muscle. The present study was designed to determine the effect of PRC on the metabolic profile of C2C12 myotubes. Overexpression of full-length PRC increased PRC gene expression by 2.7 ± 0.3-fold and protein content by 108 ± 5.3%. This modest elevation in PRC resulted in an increased rate of myoblast proliferation (61.5 ± 2.7%) and resulted in myotubes characterized by increased MyoD (18.2 ± 0.52%) and myosin heavy chain (15.4 ± 3.13%) protein. PRC overexpressing myotubes showed increases in mRNA for some-COX4 (2.6 ± 0.18-fold), ATP5B (2.7 ± 0.34-fold) cytochrome c (5.1 ± 0.68-fold)-but not all, MTCO1 (0.61 ± 0.18-fold) and HAD (0.98 ± 0.36-fold) mitochondrial genes, as well as a significant increase in cytochrome-c (28.7 ± 7.02%) protein content. The enzyme activity of the electron transport chain (ETC) complex IV (3.7 ± 0.01-fold) and citrate synthase (2.1 ± 0.14-fold) was increased by PRC, as was the mtDNA:nucDNA ratio (11 ± 0.3%). PRC increased cellular respiration (142%), basal (197%) and insulin-stimulated (253%) glucose uptake, as well as palmitate uptake (28.6 ± 3.31%) and oxidation (31.1 ± 2.17%). Associated with these changes in function, PRC overexpression increased GLUT4 mRNA (4.5 ± 0.22-fold) and protein (13.8 ± 2.08%) and CPT1 protein (28.9 ± 4.23%). Electrical stimulation of C2C12 myotubes resulted in a transient increase in PRC mRNA that was smaller (2.1 ± 0.3-fold vs. 4.4 ± 0.23-fold) and occurred earlier (3 h vs. 6 h) than PGC-1α. Collectively, our data show that PRC promotes skeletal muscle myogenesis and metabolism in vitro, thus identifying PRC as a functional skeletal muscle coactivator capable of regulating mitochondrial substrate utilization and respiration.
Figures


References
-
- Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, Ma Y, Chin S, Spiegelman BM. The transcriptional coactivator PGC-1β drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab 5: 35–46, 2007 - PubMed
-
- Baar K, Song Z, Semenkovich CF, Jones TE, Han DH, Nolte LA, Ojuka EO, Chen M, Holloszy JO. Skeletal muscle overexpression of nuclear respiratory factor 1 increases glucose transport capacity. FASEB J 17: 1666–1673, 2003 - PubMed
-
- Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 16: 1879–1886, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

