Cellular content of UDP-N-acetylhexosamines controls hyaluronan synthase 2 expression and correlates with O-linked N-acetylglucosamine modification of transcription factors YY1 and SP1
- PMID: 21795679
- PMCID: PMC3190925
- DOI: 10.1074/jbc.M111.265637
Cellular content of UDP-N-acetylhexosamines controls hyaluronan synthase 2 expression and correlates with O-linked N-acetylglucosamine modification of transcription factors YY1 and SP1
Abstract
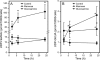
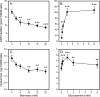
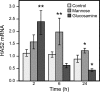
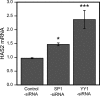
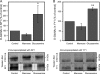
Hyaluronan, a high molecular mass polysaccharide on the vertebrate cell surface and extracellular matrix, is produced at the plasma membrane by hyaluronan synthases using UDP-GlcNAc and UDP-GlcUA as substrates. The availability of these UDP-sugar substrates can limit the synthesis rate of hyaluronan. In this study, we show that the cellular level of UDP-HexNAc also controls hyaluronan synthesis by modulating the expression of HAS2 (hyaluronan synthase 2). Increasing UDP-HexNAc in HaCaT keratinocytes by adding glucosamine down-regulated HAS2 gene expression, whereas a decrease in UDP-HexNAc, realized by mannose treatment or siRNA for GFAT1 (glutamine:fructose-6-phosphate amidotransferase 1), enhanced expression of the gene. Tracing the UDP-HexNAc-initiated signal to the HAS2 promoter revealed no change in the binding of STAT3, NF-κB, and cAMP response element-binding protein, shown previously to mediate growth factor and cytokine signals on HAS2 expression. Instead, altered binding of SP1 and YY1 to the promoter correlated with cellular UDP-HexNAc content and inhibition of HAS2 expression. siRNA silencing of YY1 and SP1 confirmed their inhibitory effects on HAS2 expression. Reduced and increased levels of O-GlcNAc-modified SP1 and YY1 proteins were associated with stimulation or inhibition of HAS2 expression, respectively. Our data are consistent with the hypothesis that, by regulating the level of protein O-GlcNAc modifications, cellular UDP-HexNAc content controls HAS2 transcription and decreases the effects on hyaluronan synthesis that would result from cellular fluctuations of this substrate.
Figures
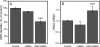
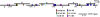
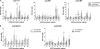
References
-
- Toole B. P. (2004) Nat. Rev. Cancer 4, 528–539 - PubMed
-
- Weigel P. H., DeAngelis P. L. (2007) J. Biol. Chem. 282, 36777–36781 - PubMed
-
- Kuroda K., Utani A., Hamasaki Y., Shinkai H. (2001) J. Dermatol. Sci. 26, 156–160 - PubMed
-
- Yamada Y., Itano N., Hata K., Ueda M., Kimata K. (2004) J. Invest. Dermatol. 122, 631–639 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

