Nucleobase transport by human equilibrative nucleoside transporter 1 (hENT1)
- PMID: 21795683
- PMCID: PMC3173155
- DOI: 10.1074/jbc.M111.236117
Nucleobase transport by human equilibrative nucleoside transporter 1 (hENT1)
Abstract
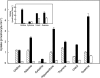
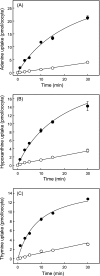
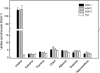
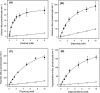
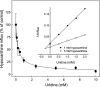
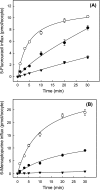
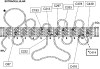
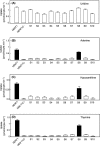
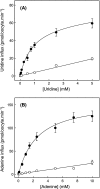
The human equilibrative nucleoside transporters hENT1 and hENT2 (each with 456 residues) are 40% identical in amino acid sequence and contain 11 putative transmembrane helices. Both transport purine and pyrimidine nucleosides and are distinguished functionally by a difference in sensitivity to inhibition by nanomolar concentrations of nitrobenzylmercaptopurine ribonucleoside (NBMPR), hENT1 being NBMPR-sensitive. Previously, we used heterologous expression in Xenopus oocytes to demonstrate that recombinant hENT2 and its rat ortholog rENT2 also transport purine and pyrimidine bases, h/rENT2 representing the first identified mammalian nucleobase transporter proteins (Yao, S. Y., Ng, A. M., Vickers, M. F., Sundaram, M., Cass, C. E., Baldwin, S. A., and Young, J. D. (2002) J. Biol. Chem. 277, 24938-24948). The same study also revealed lower, but significant, transport of hypoxanthine by h/rENT1. In the present investigation, we have used the enhanced Xenopus oocyte expression vector pGEMHE to demonstrate that hENT1 additionally transports thymine and adenine and, to a lesser extent, uracil and guanine. Fluxes of hypoxanthine, thymine, and adenine by hENT1 were saturable and inhibited by NBMPR. Ratios of V(max) (pmol/oocyte · min(-1)):K(m) (mm), a measure of transport efficiency, were 86, 177, and 120 for hypoxantine, thymine, and adenine, respectively, compared with 265 for uridine. Hypoxanthine influx was competitively inhibited by uridine, indicating common or overlapping nucleobase and nucleoside permeant binding pockets, and the anticancer nucleobase drugs 5-fluorouracil and 6-mercaptopurine were also transported. Nucleobase transport activity was absent from an engineered cysteine-less version hENT1 (hENT1C-) in which all 10 endogenous cysteine residues were mutated to serine. Site-directed mutagenesis identified Cys-414 in transmembrane helix 10 of hENT1 as the residue conferring nucleobase transport activity to the wild-type transporter.
Figures


References
-
- Zhang J., Visser F., King K. M., Baldwin S. A., Young J. D., Cass C. E. (2007) Metastasis Rev. 26, 85–110 - PubMed
-
- King A. E., Ackley M. A., Cass C. E., Young J. D., Baldwin S. A. (2006) Trends Pharmacol. Sci. 27, 416–425 - PubMed
-
- Cabrita M. A., Baldwin S. A., Young J. D., Cass C. E. (2002) Biochem. Cell. Biol. 80, 623–638 - PubMed
-
- Young J. D., Cheeseman C. I., Mackey J. R., Cass C. E., Baldwin S. A. (2000) in Current Topics in Membranes (Barrett K. E., Donowitz M. eds.) pp. 329–378, Academic Press, San Diego, CA
-
- Griffith D. A., Jarvis S. M. (1996) Biochim. Biophys. Acta 1286, 153–181 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

