Magnetic nanoparticle biodistribution following intratumoral administration
- PMID: 21795772
- PMCID: PMC3158492
- DOI: 10.1088/0957-4484/22/34/345101
Magnetic nanoparticle biodistribution following intratumoral administration
Abstract
Recently, heat generated by iron oxide nanoparticles (IONPs) stimulated by an alternating magnetic field (AMF) has shown promise in the treatment of cancer. To determine the mechanism of nanoparticle-induced cytotoxicity, the physical association of the cancer cells and the nanoparticles must be determined. We have used transmission electron microscopy (TEM) to define the time dependent cellular uptake of intratumorally administered dextran-coated, core-shell configuration IONP having a mean hydrodynamic diameter of 100-130 nm in a murine breast adenocarcinoma cell line (MTG-B) in vivo. Tumors averaging volumes of 115 mm3 were injected with iron oxide nanoparticles. The tumors were then excised and fixed for TEM at time 0.1-120 h post-IONP injection. Intracellular uptake of IONPs was 5.0, 48.8 and 91.1% uptake at one, 2 and 4 h post-injection of IONPs, respectively. This information is essential for the effective use of IONP hyperthermia in cancer treatment.
Figures


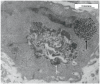
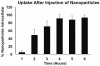

References
-
- Storm FK, Baker HW, Scanlon EF, Plenk HP, Meadows PM, Cohen SC, et al. Magnetic-induction hyperthermia. Results of a 5-year multi-institutional national cooperative trial in advanced cancer patients. Cancer. 1985;55(11):2677–2687. - PubMed
-
- Horsman MR, Overgaard J. Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol. 2007;19:418–426. - PubMed
-
- Gazeau F, Levy M, Wilhelm C. Optimizing magnetic nanoparticle design for nanothermotherapy. Nanomedicine. 2008;3(6):831–844. - PubMed
-
- Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in Medicine: Therapeutic Applications and Developments. Clin Pharmacol Ther. 2008;83(5):761–769. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
