Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory
- PMID: 21796108
- PMCID: PMC3176574
- DOI: 10.1038/npp.2011.127
Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory
Abstract
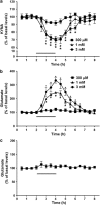
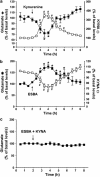
Kynurenic acid (KYNA), an astrocyte-derived metabolite, antagonizes the α7 nicotinic acetylcholine receptor (α7nAChR) and, possibly, the glycine co-agonist site of the NMDA receptor at endogenous brain concentrations. As both receptors are involved in cognitive processes, KYNA elevations may aggravate, whereas reductions may improve, cognitive functions. We tested this hypothesis in rats by examining the effects of acute up- or downregulation of endogenous KYNA on extracellular glutamate in the hippocampus and on performance in the Morris water maze (MWM). Applied directly by reverse dialysis, KYNA (30-300 nM) reduced, whereas the specific kynurenine aminotransferase-II inhibitor (S)-4-(ethylsulfonyl)benzoylalanine (ESBA; 0.3-3 mM) raised, extracellular glutamate levels in the hippocampus. Co-application of KYNA (100 nM) with ESBA (1 mM) prevented the ESBA-induced glutamate increase. Comparable effects on hippocampal glutamate levels were seen after intra-cerebroventricular (i.c.v.) application of the KYNA precursor kynurenine (1 mM, 10 μl) or ESBA (10 mM, 10 μl), respectively. In separate animals, i.c.v. treatment with kynurenine impaired, whereas i.c.v. ESBA improved, performance in the MWM. I.c.v. co-application of KYNA (10 μM) eliminated the pro-cognitive effects of ESBA. Collectively, these studies show that KYNA serves as an endogenous modulator of extracellular glutamate in the hippocampus and regulates hippocampus-related cognitive function. Our results suggest that pharmacological interventions leading to acute reductions in hippocampal KYNA constitute an effective strategy for cognitive improvement. This approach might be especially useful in the treatment of cognitive deficits in neurological and psychiatric diseases that are associated with increased brain KYNA levels.
Figures




References
-
- Abbott A, Roberts BM, Turner L, Campbell DW, Schaffer CL, Campbell BM, et al. Inhibition of kynurenine aminotransferase II (KAT II) protects against ketamine-induced cognitive impairment and improves spatial working memory. Soc Neurosci Abstr. 2010;35:472.18.
-
- Ahlander M, Misane I, Schott PA, Ogren SO. A behavioral analysis of the spatial learning deficit induced by the NMDA receptor antagonist MK-801 (dizocilpine) in the rat. Neuropsychopharmacology. 1999;21:414–426. - PubMed
-
- Bannerman DM, Rawlins JN, Good MA. The drugs don't work-or do they? Pharmacological and transgenic studies of the contribution of NMDA and GluR-A-containing AMPA receptors to hippocampal-dependent memory. Psychopharmacology (Berl) 2006;188:552–566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

