A model of calcium activation of the cardiac thin filament
- PMID: 21797264
- PMCID: PMC3165030
- DOI: 10.1021/bi200506k
A model of calcium activation of the cardiac thin filament
Abstract
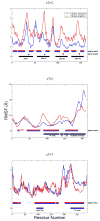
The cardiac thin filament regulates actomyosin interactions through calcium-dependent alterations in the dynamics of cardiac troponin and tropomyosin. Over the past several decades, many details of the structure and function of the cardiac thin filament and its components have been elucidated. We propose a dynamic, complete model of the thin filament that encompasses known structures of cardiac troponin, tropomyosin, and actin and show that it is able to capture key experimental findings. By performing molecular dynamics simulations under two conditions, one with calcium bound and the other without calcium bound to site II of cardiac troponin C (cTnC), we found that subtle changes in structure and protein contacts within cardiac troponin resulted in sweeping changes throughout the complex that alter tropomyosin (Tm) dynamics and cardiac troponin--actin interactions. Significant calcium-dependent changes in dynamics occur throughout the cardiac troponin complex, resulting from the combination of the following: structural changes in the N-lobe of cTnC at and adjacent to sites I and II and the link between them; secondary structural changes of the cardiac troponin I (cTnI) switch peptide, of the mobile domain, and in the vicinity of residue 25 of the N-terminus; secondary structural changes in the cardiac troponin T (cTnT) linker and Tm-binding regions; and small changes in cTnC-cTnI and cTnT-Tm contacts. As a result of these changes, we observe large changes in the dynamics of the following regions: the N-lobe of cTnC, the mobile domain of cTnI, the I-T arm, the cTnT linker, and overlapping Tm. Our model demonstrates a comprehensive mechanism for calcium activation of the cardiac thin filament consistent with previous, independent experimental findings. This model provides a valuable tool for research into the normal physiology of cardiac myofilaments and a template for studying cardiac thin filament mutations that cause human cardiomyopathies.
Figures



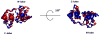




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

