T-tubule biogenesis and triad formation in skeletal muscle and implication in human diseases
- PMID: 21797990
- PMCID: PMC3156648
- DOI: 10.1186/2044-5040-1-26
T-tubule biogenesis and triad formation in skeletal muscle and implication in human diseases
Abstract
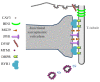
In skeletal muscle, the excitation-contraction (EC) coupling machinery mediates the translation of the action potential transmitted by the nerve into intracellular calcium release and muscle contraction. EC coupling requires a highly specialized membranous structure, the triad, composed of a central T-tubule surrounded by two terminal cisternae from the sarcoplasmic reticulum. While several proteins located on these structures have been identified, mechanisms governing T-tubule biogenesis and triad formation remain largely unknown. Here, we provide a description of triad structure and plasticity and review the role of proteins that have been linked to T-tubule biogenesis and triad formation and/or maintenance specifically in skeletal muscle: caveolin 3, amphiphysin 2, dysferlin, mitsugumins, junctophilins, myotubularin, ryanodine receptor, and dihydhropyridine Receptor. The importance of these proteins in triad biogenesis and subsequently in muscle contraction is sustained by studies on animal models and by the direct implication of most of these proteins in human myopathies.
Figures



References
-
- Fosset M, Jaimovich E, Delpont E, Lazdunski M. [3H]nitrendipine receptors in skeletal muscle. J Biol Chem. 1983;258:6086–6092. - PubMed
-
- Inui M, Saito A, Fleischer S. Isolation of the ryanodine receptor from cardiac sarcoplasmic reticulum and identity with the feet structures. J Biol Chem. 1987;262:15637–15642. - PubMed
LinkOut - more resources
Full Text Sources
Medical

