Safety and feasibility of third-party multipotent adult progenitor cells for immunomodulation therapy after liver transplantation--a phase I study (MISOT-I)
- PMID: 21798013
- PMCID: PMC3166276
- DOI: 10.1186/1479-5876-9-124
Safety and feasibility of third-party multipotent adult progenitor cells for immunomodulation therapy after liver transplantation--a phase I study (MISOT-I)
Abstract
Background: Liver transplantation is the definitive treatment for many end-stage liver diseases. However, the life-long immunosuppression needed to prevent graft rejection causes clinically significant side effects. Cellular immunomodulatory therapies may allow the dose of immunosuppressive drugs to be reduced. In the current protocol, we propose to complement immunosuppressive pharmacotherapy with third-party multipotent adult progenitor cells (MAPCs), a culture-selected population of adult adherent stem cells derived from bone marrow that has been shown to display potent immunomodulatory and regenerative properties. In animal models, MAPCs reduce the need for pharmacological immunosuppression after experimental solid organ transplantation and regenerate damaged organs.
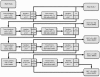
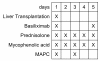
Methods: Patients enrolled in this phase I, single-arm, single-center safety and feasibility study (n = 3-24) will receive 2 doses of third-party MAPCs after liver transplantation, on days 1 and 3, in addition to a calcineurin-inhibitor-free "bottom-up" immunosuppressive regimen with basiliximab, mycophenolic acid, and steroids. The study objective is to evaluate the safety and clinical feasibility of MAPC administration in this patient cohort. The primary endpoint of the study is safety, assessed by standardized dose-limiting toxicity events. One secondary endpoint is the time until first biopsy-proven acute rejection, in order to collect first evidence of efficacy. Dose escalation (150, 300, 450, and 600 million MAPCs) will be done according to a 3 + 3 classical escalation design (4 groups of 3-6 patients each).
Discussion: If MAPCs are safe for patients undergoing liver transplantation in this study, a phase II/III trial will be conducted to assess their clinical efficacy.
Figures


Similar articles
-
First-in-Human Case Study: Multipotent Adult Progenitor Cells for Immunomodulation After Liver Transplantation.Stem Cells Transl Med. 2015 Aug;4(8):899-904. doi: 10.5966/sctm.2015-0002. Epub 2015 Jun 3. Stem Cells Transl Med. 2015. PMID: 26041737 Free PMC article. Clinical Trial.
-
Heart grafts tolerized through third-party multipotent adult progenitor cells can be retransplanted to secondary hosts with no immunosuppression.Stem Cells Transl Med. 2013 Aug;2(8):595-606. doi: 10.5966/sctm.2012-0166. Epub 2013 Jul 8. Stem Cells Transl Med. 2013. PMID: 23836805 Free PMC article.
-
Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial.Lancet Neurol. 2017 May;16(5):360-368. doi: 10.1016/S1474-4422(17)30046-7. Epub 2017 Mar 17. Lancet Neurol. 2017. PMID: 28320635 Clinical Trial.
-
ISA 247: trans-ISA 247, trans-R 1524, ISA(TX)247, ISAtx 247, ISATx247, LX 211, LX211, R 1524, R-1524.Drugs R D. 2007;8(2):103-12. doi: 10.2165/00126839-200708020-00005. Drugs R D. 2007. PMID: 17324008 Review.
-
A Comparison of Phenotypic and Functional Properties of Mesenchymal Stromal Cells and Multipotent Adult Progenitor Cells.Front Immunol. 2019 Aug 28;10:1952. doi: 10.3389/fimmu.2019.01952. eCollection 2019. Front Immunol. 2019. PMID: 31555259 Free PMC article. Review.
Cited by
-
First-in-Human Case Study: Multipotent Adult Progenitor Cells for Immunomodulation After Liver Transplantation.Stem Cells Transl Med. 2015 Aug;4(8):899-904. doi: 10.5966/sctm.2015-0002. Epub 2015 Jun 3. Stem Cells Transl Med. 2015. PMID: 26041737 Free PMC article. Clinical Trial.
-
The Delivery of Multipotent Adult Progenitor Cells to Extended Criteria Human Donor Livers Using Normothermic Machine Perfusion.Front Immunol. 2020 Jun 25;11:1226. doi: 10.3389/fimmu.2020.01226. eCollection 2020. Front Immunol. 2020. PMID: 32714318 Free PMC article.
-
N-acetylcysteine prevents oxidized low-density lipoprotein-induced reduction of MG53 and enhances MG53 protective effect on bone marrow stem cells.J Cell Mol Med. 2020 Jan;24(1):886-898. doi: 10.1111/jcmm.14798. Epub 2019 Nov 19. J Cell Mol Med. 2020. PMID: 31742908 Free PMC article.
-
Human Bone Marrow- and Adipose Tissue-derived Mesenchymal Stromal Cells are Immunosuppressive In vitro and in a Humanized Allograft Rejection Model.J Stem Cell Res Ther. 2013 Nov 25;Suppl 6(1):20780. doi: 10.4172/2157-7633.S6-001. J Stem Cell Res Ther. 2013. PMID: 24672744 Free PMC article.
-
MSC-based therapies in solid organ transplantation.Hepatol Int. 2014 Apr;8(2):179-84. doi: 10.1007/s12072-013-9509-1. Epub 2014 Jan 4. Hepatol Int. 2014. PMID: 26202500
References
-
- Cattaneo D, Perico N, Gaspari F, Remuzzi G. Nephrotoxic aspects of cyclosporine. TransplantProc. 2004;36:234S–239S. - PubMed
-
- Christians U, Kohlhaw K, Budniak J, Bleck JS, Schottmann R, Schlitt HJ, Almeida VM, Deters M, Wonigeit K, Pichlmayr R. Ciclosporin metabolite pattern in blood and urine of liver graft recipients. I. Association of ciclosporin metabolites with nephrotoxicity. EurJClinPharmacol. 1991;41:285–290. - PubMed
-
- Mueller AR, Platz KP, Schattenfroh N, Bechstein WO, Christe W, Neuhaus P. Neurotoxicity after orthotopic liver transplantation in cyclosporin A- and FK 506-treated patients. TransplInt. 1994;7(Suppl 1):S37–S42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

