Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models
- PMID: 21798082
- PMCID: PMC3143906
- DOI: 10.1186/2044-5040-1-4
Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models
Abstract
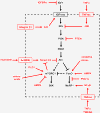
A highly conserved signaling pathway involving insulin-like growth factor 1 (IGF1), and a cascade of intracellular components that mediate its effects, plays a major role in the regulation of skeletal muscle growth. A central component in this cascade is the kinase Akt, also called protein kinase B (PKB), which controls both protein synthesis, via the kinases mammalian target of rapamycin (mTOR) and glycogen synthase kinase 3β (GSK3β), and protein degradation, via the transcription factors of the FoxO family. In this paper, we review the composition and function of this pathway in skeletal muscle fibers, focusing on evidence obtained in vivo by transgenic and knockout models and by muscle transient transfection experiments. Although this pathway is essential for muscle growth during development and regeneration, its role in adult muscle response to mechanical load is less clear. A full understanding of the operation of this pathway could help to design molecularly targeted therapeutics aimed at preventing muscle wasting, which occurs in a variety of pathologic contexts and in the course of aging.
Figures



Similar articles
-
PI3 kinase regulation of skeletal muscle hypertrophy and atrophy.Curr Top Microbiol Immunol. 2010;346:267-78. doi: 10.1007/82_2010_78. Curr Top Microbiol Immunol. 2010. PMID: 20593312 Review.
-
A novel PKB/Akt inhibitor, MK-2206, effectively inhibits insulin-stimulated glucose metabolism and protein synthesis in isolated rat skeletal muscle.Biochem J. 2012 Oct 1;447(1):137-47. doi: 10.1042/BJ20120772. Biochem J. 2012. PMID: 22793019
-
Burn injury impairs insulin-stimulated Akt/PKB activation in skeletal muscle.Am J Physiol Endocrinol Metab. 2005 Mar;288(3):E585-91. doi: 10.1152/ajpendo.00321.2004. Epub 2004 Nov 9. Am J Physiol Endocrinol Metab. 2005. PMID: 15536206
-
Testosterone regulation of Akt/mTORC1/FoxO3a signaling in skeletal muscle.Mol Cell Endocrinol. 2013 Jan 30;365(2):174-86. doi: 10.1016/j.mce.2012.10.019. Epub 2012 Oct 29. Mol Cell Endocrinol. 2013. PMID: 23116773 Free PMC article.
-
Triggers and mechanisms of skeletal muscle wasting in chronic obstructive pulmonary disease.Int J Biochem Cell Biol. 2013 Oct;45(10):2245-56. doi: 10.1016/j.biocel.2013.06.015. Epub 2013 Jul 1. Int J Biochem Cell Biol. 2013. PMID: 23827718 Review.
Cited by
-
Activation of IGF-1 pathway and suppression of atrophy related genes are involved in Epimedium extract (icariin) promoted C2C12 myotube hypertrophy.Sci Rep. 2021 May 24;11(1):10790. doi: 10.1038/s41598-021-89039-0. Sci Rep. 2021. PMID: 34031457 Free PMC article.
-
Murine models of atrophy, cachexia, and sarcopenia in skeletal muscle.Biochim Biophys Acta. 2013 Sep;1832(9):1410-20. doi: 10.1016/j.bbadis.2013.03.011. Epub 2013 Mar 20. Biochim Biophys Acta. 2013. PMID: 23523469 Free PMC article. Review.
-
Overload-induced skeletal muscle hypertrophy is not impaired in STZ-diabetic rats.Physiol Rep. 2015 Jul;3(7):e12457. doi: 10.14814/phy2.12457. Physiol Rep. 2015. PMID: 26197932 Free PMC article.
-
Skeletal Muscle Loss during Multikinase Inhibitors Therapy: Molecular Pathways, Clinical Implications, and Nutritional Challenges.Nutrients. 2020 Oct 12;12(10):3101. doi: 10.3390/nu12103101. Nutrients. 2020. PMID: 33053632 Free PMC article. Review.
-
Differential induction of muscle atrophy pathways in two mouse models of spinal muscular atrophy.Sci Rep. 2016 Jun 28;6:28846. doi: 10.1038/srep28846. Sci Rep. 2016. PMID: 27349908 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

