Aberrant repair and fibrosis development in skeletal muscle
- PMID: 21798099
- PMCID: PMC3156644
- DOI: 10.1186/2044-5040-1-21
Aberrant repair and fibrosis development in skeletal muscle
Abstract
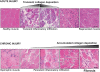
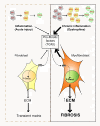
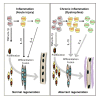
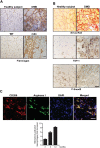
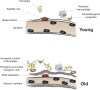
The repair process of damaged tissue involves the coordinated activities of several cell types in response to local and systemic signals. Following acute tissue injury, infiltrating inflammatory cells and resident stem cells orchestrate their activities to restore tissue homeostasis. However, during chronic tissue damage, such as in muscular dystrophies, the inflammatory-cell infiltration and fibroblast activation persists, while the reparative capacity of stem cells (satellite cells) is attenuated. Abnormal dystrophic muscle repair and its end stage, fibrosis, represent the final common pathway of virtually all chronic neurodegenerative muscular diseases. As our understanding of the pathogenesis of muscle fibrosis has progressed, it has become evident that the muscle provides a useful model for the regulation of tissue repair by the local microenvironment, showing interplay among muscle-specific stem cells, inflammatory cells, fibroblasts and extracellular matrix components of the mammalian wound-healing response. This article reviews the emerging findings of the mechanisms that underlie normal versus aberrant muscle-tissue repair.
Figures
References
-
- Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiological reviews. 2002;82:291–329. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

