Long-term efficacy and safety outcomes with OROS-MPH in adults with ADHD
- PMID: 21798108
- PMCID: PMC3243903
- DOI: 10.1017/S1461145711001131
Long-term efficacy and safety outcomes with OROS-MPH in adults with ADHD
Abstract
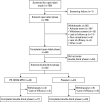
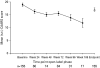
Methylphenidate (MPH) is widely prescribed for adults with attention deficit hyperactivity disorder (ADHD), but data on long-term treatment and maintenance of effect are lacking. Osmotic release oral system-methylphenidate (OROS-MPH) was evaluated in a 52-wk open-label study in subjects who had previously completed a short-term placebo-controlled trial and short-term open-label extension. Efficacy was assessed using the investigator- and subject-rated Conners' Adult ADHD Rating Scales (CAARS:O-SV and CAARS:S-S), and the Clinical Global Impression - Severity (CGI-S), Sheehan Disability Scale (SDS) and Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q). Subjects completing ≥52 wk of treatment were eligible for a 4-wk randomized, placebo-controlled withdrawal phase in which loss of treatment effect was assessed using CAARS:O-SV and CGI-S. In the open-label phase (n=156), mean CAARS:O-SV score decreased from baseline by 1.9±7.8 (p<0.01), and small, statistically significant improvements from baseline were observed for CAARS:S-S, CGI-S and SDS. In the double-blind phase (OROS-MPH, n=23; placebo, n=22), CAARS:O-SV increased from double-blind baseline in the OROS-MPH and placebo arms (4.0±7.6 vs. 6.5±7.8, not statistically significant). Long-term OROS-MPH treatment was well tolerated, and there was no evidence of withdrawal or rebound after discontinuation. In conclusion, the short-term benefits of OROS-MPH continue during long-term open-label treatment. Maintenance of efficacy in a placebo-controlled withdrawal design remains to be confirmed in larger patient populations.
Trial registration: ClinicalTrials.gov NCT00307684.
Figures
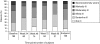


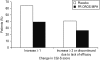
References
-
- Adler LA, Orman C, Starr HL, Silber S. et al. Long-term safety of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder: an open-label, dose-titration, 1-year study. Journal of Clinical Psychopharmacology. 2011;31:108–114. - PubMed
-
- Adler LA, Spencer TJ, Milton DR, Moore RJ. et al. Long-term, open-label study of the safety and efficacy of atomoxetine in adults with attention-deficit/hyperactivity disorder: an interim analysis. Journal of Clinical Psychiatry. 2005;66:294–299. - PubMed
-
- Adler LA, Zimmerman B, Starr HL, Silber S. et al. Efficacy and safety of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, double-blind, parallel group, dose-escalation study. Journal of Clinical Psychopharmacology. 2009;29:239–247. - PubMed
-
- Bejerot S, Rydén EM, Arlinde CM. Two-year outcome of treatment with central stimulant medication in adult attention-deficit/hyperactivity disorder: a prospective study. Journal of Clinical Psychiatry. 2010;71:1590–1597. - PubMed
-
- Biederman J, Mick E, Surman C, Doyle R. et al. A randomized, 3-phase, 34-week, double-blind, long-term efficacy study of osmotic-release oral system-methylphenidate in adults with attention-deficit/hyperactivity disorder. Journal of Clinical Psychopharmacology. 2010;30:549–553. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

