RNA mimics of green fluorescent protein
- PMID: 21798953
- PMCID: PMC3314379
- DOI: 10.1126/science.1207339
RNA mimics of green fluorescent protein
Abstract
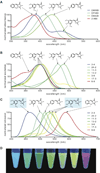
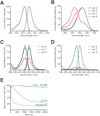
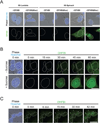
Green fluorescent protein (GFP) and its derivatives have transformed the use and analysis of proteins for diverse applications. Like proteins, RNA has complex roles in cellular function and is increasingly used for various applications, but a comparable approach for fluorescently tagging RNA is lacking. Here, we describe the generation of RNA aptamers that bind fluorophores resembling the fluorophore in GFP. These RNA-fluorophore complexes create a palette that spans the visible spectrum. An RNA-fluorophore complex, termed Spinach, resembles enhanced GFP and emits a green fluorescence comparable in brightness with fluorescent proteins. Spinach is markedly resistant to photobleaching, and Spinach fusion RNAs can be imaged in living cells. These RNA mimics of GFP provide an approach for genetic encoding of fluorescent RNAs.
Figures

References
-
- Chudakov DM, Matz MV, Lukyanov S, Lukyanov KA. Fluorescent Proteins and Their Applications in Imaging Living Cells and Tissues. Physiol. Rev. 2010;90:1103. - PubMed
-
- Ward WW, Bokman SH. Reversible denaturation of Aequorea green-fluorescent protein: physical separation and characterization of the renatured protein. Biochemistry (Mosc) 1982;21:4535. - PubMed
-
- Meech S. Excited state reactions in fluorescent proteins. Chem. Soc. Rev. 2009 - PubMed
-
- Babendure J, Adams S, Tsien R. Aptamers switch on fluorescence of triphenylmethane dyes. J. Am. Chem. Soc. 2003;125:14716. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

