Iridoid-specific glucosyltransferase from Gardenia jasminoides
- PMID: 21799001
- PMCID: PMC3173207
- DOI: 10.1074/jbc.M111.242586
Iridoid-specific glucosyltransferase from Gardenia jasminoides
Abstract
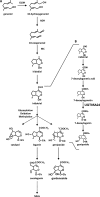
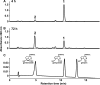
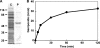
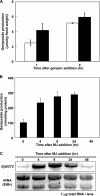
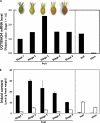
Iridoids are one of the most widely distributed secondary metabolites in higher plants. They are pharmacologically active principles in various medicinal plants and key intermediates in the biosynthesis of monoterpenoid indole alkaloids as well as quinoline alkaloids. Although most iridoids are present as 1-O-glucosides, the glucosylation step in the biosynthetic pathway has remained obscure. We isolated a cDNA coding for UDP-glucose:iridoid glucosyltransferase (UGT85A24) from Gardenia jasminoides. UGT85A24 preferentially glucosylated the 1-O-hydroxyl group of 7-deoxyloganetin and genipin but exhibited only weak activity toward loganetin and no activity toward 7-deoxyloganetic acid. This suggests that, in the biosynthetic pathway of geniposide, a major iridoid compound in G. jasminoides, glucosylation occurs after methylation of 7-deoxyloganetic acid. UGT85A24 showed negligible activity toward any acceptor substrates other than iridoid aglycones. Thus, UGT85A24 has a remarkable specificity for iridoid aglycones. The mRNA level of UGT85A24 overlaps with the marked increase in genipin glucosylation activity in the methyl jasmonate-treated cell cultures of G. jasminoides and is related to iridoid accumulation in G. jasminoides fruits.
Figures


References
-
- Dinda B., Debnath S., Harigaya Y. (2007) Chem. Pharm. Bull. 55, 159–222 - PubMed
-
- Harvey J. A., van Nouhuys S., Biere A. (2005) J. Chem. Ecol. 31, 287–302 - PubMed
-
- El-Sayed M., Verpoorte R. (2007) Phytochem. Rev. 6, 277–305
-
- Ghisalberti E. L. (1998) Phytomedicine 5, 147–163 - PubMed
-
- Dinda B., Debnath S., Harigaya Y. (2007) Chem. Pharm. Bull. 55, 689–728 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

