Conformational regulation of urokinase receptor function: impact of receptor occupancy and epitope-mapped monoclonal antibodies on lamellipodia induction
- PMID: 21799009
- PMCID: PMC3190949
- DOI: 10.1074/jbc.M111.220087
Conformational regulation of urokinase receptor function: impact of receptor occupancy and epitope-mapped monoclonal antibodies on lamellipodia induction
Abstract
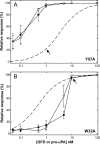
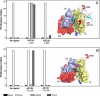
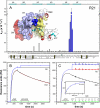
The urokinase-type plasminogen activator receptor (uPAR) is a glycolipid-anchored membrane protein with an established role in focalizing uPA-mediated plasminogen activation on cell surfaces. Distinct from this function, uPAR also modulates cell adhesion and migration on vitronectin-rich matrices. Although uPA and vitronectin engage structurally distinct binding sites on uPAR, they nonetheless cooperate functionally, as uPA binding potentiates uPAR-dependent induction of lamellipodia on vitronectin matrices. We now present data advancing the possibility that it is the burial of the β-hairpin in uPA per se into the hydrophobic ligand binding cavity of uPAR that modulates the function of this receptor. Based on these data, we now propose a model in which the inherent interdomain mobility in uPAR plays a major role in modulating its function. Particularly one uPAR conformation, which is stabilized by engagement of the β-hairpin in uPA, favors the proper assembly of an active, compact receptor structure that stimulates lamellipodia induction on vitronectin. This molecular model has wide implications for drug development targeting uPAR function.
Figures





References
-
- Friedl P., Gilmour D. (2009) Nat. Rev. Mol. Cell Biol. 10, 445–457 - PubMed
-
- Friedl P., Wolf K. (2009) Cancer Metastasis Rev. 28, 129–135 - PubMed
-
- Smith H. W., Marshall C. J. (2010) Nat. Rev. Mol. Cell Biol. 11, 23–36 - PubMed
-
- Kjaergaard M., Hansen L. V., Jacobsen B., Gårdsvoll H., Ploug M. (2008) Front. Biosci. 13, 5441–5461 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

