Rod phosphodiesterase-6 (PDE6) catalytic subunits restore cone function in a mouse model lacking cone PDE6 catalytic subunit
- PMID: 21799013
- PMCID: PMC3190866
- DOI: 10.1074/jbc.M111.259101
Rod phosphodiesterase-6 (PDE6) catalytic subunits restore cone function in a mouse model lacking cone PDE6 catalytic subunit
Abstract
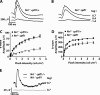
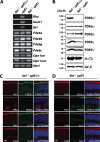
Rod and cone photoreceptor neurons utilize discrete PDE6 enzymes that are crucial for phototransduction. Rod PDE6 is composed of heterodimeric catalytic subunits (αβ), while the catalytic core of cone PDE6 (α') is a homodimer. It is not known if variations between PDE6 subunits preclude rod PDE6 catalytic subunits from coupling to the cone phototransduction pathway. To study this issue, we generated a cone-dominated mouse model lacking cone PDE6 (Nrl(-/-) cpfl1). In this animal model, using several independent experimental approaches, we demonstrated the expression of rod PDE6 (αβ) and the absence of cone PDE6 (α') catalytic subunits. The rod PDE6 enzyme expressed in cone cells is active and contributes to the hydrolysis of cGMP in response to light. In addition, rod PDE6 expressed in cone cells couples to the light signaling pathway to produce S-cone responses. However, S-cone responses and light-dependent cGMP hydrolysis were eliminated when the β-subunit of rod PDE6 was removed (Nrl(-/-) cpfl1 rd). We conclude that either rod or cone PDE6 can effectively couple to the cone phototransduction pathway to mediate visual signaling. Interestingly, we also found that functional PDE6 is required for trafficking of M-opsin to cone outer segments.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

