Efficient derivation of NPCs, spinal motor neurons and midbrain dopaminergic neurons from hESCs at 3% oxygen
- PMID: 21799491
- PMCID: PMC3433269
- DOI: 10.1038/nprot.2011.380
Efficient derivation of NPCs, spinal motor neurons and midbrain dopaminergic neurons from hESCs at 3% oxygen
Abstract
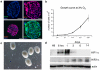
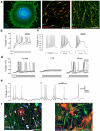
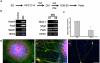
This protocol has been designed to generate neural precursor cells (NPCs) from human embryonic stem cells (hESCs) using a physiological oxygen (O(2)) level of 3% (previously termed hypoxia) and chemically defined conditions. The first stage involves suspension culture of hESC colonies at 3% O(2), where they acquire a neuroepithelial identity over a period of 2 weeks. This timescale is comparable to that observed at 20% O(2), but survival is enhanced. Sequential application of retinoic acid and purmorphamine (PM), from day 14 to day 28, directs differentiation toward spinal motor neurons. Alternatively, addition of fibroblast growth factor-8 and PM generates midbrain dopaminergic neurons. OLIG2 (encoding oligodendrocyte lineage transcription factor 2) induction in motor neuron precursors is twofold greater than that at 20% O(2), whereas EN1 (encoding engrailed homeobox 1) expression is enhanced fivefold. NPCs (at 3% O(2)) can be differentiated into all three neural lineages, and such cultures can be maintained long term in the absence of neurotrophins. The ability to generate defined cell types at 3% O(2) should represent a significant advancement for in vitro disease modeling and potentially for cell-based therapies.
Figures



References
-
- Zhao T, et al. Hypoxia-driven proliferation of embryonic neural stem/progenitor cells--role of hypoxia-inducible transcription factor-1alpha. FEBS J. 2008;275:1824–1834. - PubMed
-
- Hedlund E, Perlmann T. Neuronal cell replacement in Parkinson’s disease. J. Intern. Med. 2009;266:358–371. - PubMed
-
- Conti L, Cattaneo E. Neural stem cell systems: physiological players or in vitro entities? Nat. Rev. Neurosci. 2010;11:176–187. - PubMed
-
- Munoz-Sanjuan I, Brivanlou AH. Neural induction, the default model and embryonic stem cells. Nat. Rev. Neurosci. 2002;3:271–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

