Activation of autophagy in a rat model of retinal ischemia following high intraocular pressure
- PMID: 21799881
- PMCID: PMC3142183
- DOI: 10.1371/journal.pone.0022514
Activation of autophagy in a rat model of retinal ischemia following high intraocular pressure
Abstract
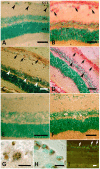
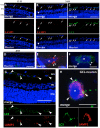
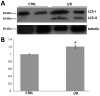
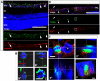
Acute primary open angle glaucoma is an optic neuropathy characterized by the elevation of intraocular pressure, which causes retinal ischemia and neuronal death. Rat ischemia/reperfusion enhances endocytosis of both horseradish peroxidase (HRP) or fluorescent dextran into ganglion cell layer (GCL) neurons 24 h after the insult. We investigated the activation of autophagy in GCL-neurons following ischemia/reperfusion, using acid phosphatase (AP) histochemistry and immunofluorescence against LC3 and LAMP1. Retinal I/R lead to the appearance of AP-positive granules and LAMP1-positive vesicles 12 and 24 h after the insult, and LC3 labelling at 24 h, and induced a consistent retinal neuron death. At 48 h the retina was negative for autophagic markers. In addition, Western Blot analysis revealed an increase of LC3 levels after damage: the increase in the conjugated, LC3-II isoform is suggestive of autophagic activity. Inhibition of autophagy by 3-methyladenine partially prevented death of neurons and reduces apoptotic markers, 24 h post-lesion. The number of neurons in the GCL decreased significantly following I/R (I/R 12.21±1.13 vs controls 19.23±1.12 cells/500 µm); this decrease was partially prevented by 3-methyladenine (17.08±1.42 cells/500 µm), which potently inhibits maturation of autophagosomes. Treatment also prevented the increase in glial fibrillary acid protein immunoreactivity elicited by I/R. Therefore, targeting autophagy could represent a novel and promising treatment for glaucoma and retinal ischemia.
Conflict of interest statement
Figures

References
-
- Ames A, 3rd, Nesbett FB. Pathophysiology of ischemic cell death: III. Role of extracellular factors. Stroke. 1983;14:233–240. - PubMed
-
- Anderson DH, Guerin CJ, Erickson PA, Stern WH, Fisher SK. Morphological recovery in the reattached retina. Invest Ophthalmol Vis Sci. 1986;27:168–183. - PubMed
-
- Arnold AC. Anterior ischemic optic neuropathy. Semin Ophthalmol. 1995;10:221–233. - PubMed
-
- Kent D, Hickey-Dwyer M, Clark D. Long-term follow-up of ischaemic retinopathy in the antiphospholipid syndrome with lupus-like disease. Eye (Lond) 14 (Pt. 2000;3A):313–317. - PubMed
-
- Smith GG, Baird CD. Survival time of retinal cells when deprived of their blood supply by increased intraocular pressure. Am J Ophthalmol. 1952;35:133–136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

