Impact of small body weight on tenofovir-associated renal dysfunction in HIV-infected patients: a retrospective cohort study of Japanese patients
- PMID: 21799928
- PMCID: PMC3143186
- DOI: 10.1371/journal.pone.0022661
Impact of small body weight on tenofovir-associated renal dysfunction in HIV-infected patients: a retrospective cohort study of Japanese patients
Abstract
Background: Treatment with tenofovir is sometimes associated with renal dysfunction. Limited information is available on this side effect in patients with small body weight, although the use of tenofovir will spread rapidly in Asia and Africa, where patients are likely to be of smaller body weight.
Methods: In a single-center cohort, Japanese patients with HIV infection who started tenofovir-containing antiretroviral therapy were retrospectively analyzed. The incidence of tenofovir-associated renal dysfunction, defined as more than 25% decrement of estimated glomerular filtration rate (eGFR) from the baseline, was determined. The effects of small body weight and body mass index (BMI) on tenofovir-associated renal dysfunction, respectively, were estimated in univariate and multivariate Cox hazards models as the primary exposure. Other possible risk factors were evaluated by univariate analysis and those found significant were entered into the multivariate analysis.
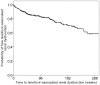
Results: The median weight of 495 patients was 63 kg. Tenofovir-related renal dysfunction occurred in 97 (19.6%) patients (incidence: 10.5 per 100 person-years). Univariate analysis showed that the incidence of tenofovir-related renal dysfunction was significantly associated with smaller body weight and BMI, respectively (per 5 kg decrement, HR = 1.23; 95% CI, 1.10-1.37; p<0.001)(per 1 kg/m(2) decrement, HR = 1.14; 95% CI, 1.05-1.23; p = 0.001). Old age, high baseline eGFR, low serum creatinine, low CD4 count, high HIV viral load, concurrent nephrotoxic drugs, hepatitis C infection, and current smoking were also associated with tenofovir-related renal dysfunction. Multivariate analysis identified small body weight as a significant risk (adjusted HR = 1.13; 95% CI, 1.01-1.27; p = 0.039), while small BMI had marginal significance (adjusted HR = 1.07; 95% CI 1.00-1.16; p = 0.058).
Conclusion: The incidence of tenofovir-associated renal dysfunction in Japanese patients was high. Small body weight was identified as an independent risk factor for tenofovir-associated renal dysfunction. Close monitoring of renal function is advocated for patients with small body weight treated with tenofovir.
Conflict of interest statement
Figures

References
-
- Gallant JE, DeJesus E, Arribas JR, Pozniak AL, Gazzard B, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354:251–260. - PubMed
-
- Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. - PubMed
-
- Izzedine H, Hulot JS, Vittecoq D, Gallant JE, Staszewski S, et al. Long-term renal safety of tenofovir disoproxil fumarate in antiretroviral-naive HIV-1-infected patients. Data from a double-blind randomized active-controlled multicentre study. Nephrol Dial Transplant. 2005;20:743–746. - PubMed
-
- Nelson MR, Katlama C, Montaner JS, Cooper DA, Gazzard B, et al. The safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: the first 4 years. AIDS. 2007;21:1273–1281. - PubMed
-
- Arribas JR, Pozniak AL, Gallant JE, Dejesus E, Gazzard B, et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz compared with zidovudine/lamivudine and efavirenz in treatment-naive patients: 144-week analysis. J Acquir Immune Defic Syndr. 2008;47:74–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

