Intracellular mechanisms of aminoglycoside-induced cytotoxicity
- PMID: 21799993
- PMCID: PMC3662252
- DOI: 10.1039/c1ib00034a
Intracellular mechanisms of aminoglycoside-induced cytotoxicity
Abstract
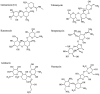
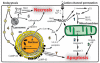
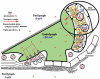
Since introduction into clinical practice over 60 years ago, aminoglycoside antibiotics remain important drugs in the treatment of bacterial infections, cystic fibrosis and tuberculosis. However, the ototoxic and nephrotoxic properties of these drugs are still a major clinical problem. Recent advances in molecular biology and biochemistry have begun to uncover the intracellular actions of aminoglycosides that lead to cytotoxicity. In this review, we discuss intracellular binding targets of aminoglycosides, highlighting specific aminoglycoside-binding proteins (HSP73, calreticulin and CLIMP-63) and their potential for triggering caspases and Bcl-2 signalling cascades that are involved in aminoglycoside-induced cytotoxicity. We also discuss potential strategies to reduce aminoglycoside cytotoxicity, which are necessary for greater bactericidal efficacy during aminoglycoside pharmacotherapy.
Figures
Similar articles
-
The relationship between the structure and toxicity of aminoglycoside antibiotics.Bioorg Med Chem Lett. 2020 Jul 1;30(13):127218. doi: 10.1016/j.bmcl.2020.127218. Epub 2020 Apr 25. Bioorg Med Chem Lett. 2020. PMID: 32360102 Free PMC article. Review.
-
Hsp70 inhibits aminoglycoside-induced hearing loss and cochlear hair cell death.Cell Stress Chaperones. 2009 Jul;14(4):427-37. doi: 10.1007/s12192-008-0097-2. Epub 2009 Jan 15. Cell Stress Chaperones. 2009. PMID: 19145477 Free PMC article.
-
[Hearing loss caused by aminoglycoside antibiotics: affect on the membrane component PIP2 in outer hair cells as the mechanism of action].HNO. 1986 Oct;34(10):417-23. HNO. 1986. PMID: 3025138 German.
-
A novel role of cytosolic protein synthesis inhibition in aminoglycoside ototoxicity.J Neurosci. 2013 Feb 13;33(7):3079-93. doi: 10.1523/JNEUROSCI.3430-12.2013. J Neurosci. 2013. PMID: 23407963 Free PMC article.
-
Molecular parameters involved in aminoglycoside nephrotoxicity.J Toxicol Environ Health. 1995 Mar;44(3):263-300. doi: 10.1080/15287399509531960. J Toxicol Environ Health. 1995. PMID: 7897692 Review.
Cited by
-
Hippo/YAP signaling pathway protects against neomycin-induced hair cell damage in the mouse cochlea.Cell Mol Life Sci. 2022 Jan 19;79(2):79. doi: 10.1007/s00018-021-04029-9. Cell Mol Life Sci. 2022. PMID: 35044530 Free PMC article.
-
Characterization of mouse Bmp5 regulatory injury element in zebrafish wound models.Bone. 2022 Feb;155:116263. doi: 10.1016/j.bone.2021.116263. Epub 2021 Nov 23. Bone. 2022. PMID: 34826632 Free PMC article.
-
Aminoglycosides: Single- or Multiple-daily Dosing? An Updated Qualitative Systematic Review of Randomized Trials on Toxicity and Efficacy.Curr Mol Med. 2024;24(11):1358-1373. doi: 10.2174/1566524023666230801160452. Curr Mol Med. 2024. PMID: 37533241
-
The relationship between the structure and toxicity of aminoglycoside antibiotics.Bioorg Med Chem Lett. 2020 Jul 1;30(13):127218. doi: 10.1016/j.bmcl.2020.127218. Epub 2020 Apr 25. Bioorg Med Chem Lett. 2020. PMID: 32360102 Free PMC article. Review.
-
Translational readthrough-promoting drugs enhance pseudoknot-mediated suppression of the stop codon at the Moloney murine leukemia virus gag–pol junction.J Gen Virol. 2015 Nov;96(11):3411-3421. doi: 10.1099/jgv.0.000284. J Gen Virol. 2015. PMID: 26382736 Free PMC article.
References
-
- Forge A, Schacht J. Audiol. Neuro-Otol. 2000;5:3–22. - PubMed
-
- Edson RS, Terrell CL. Mayo Clin. Proc. 1999;74:519–528. - PubMed
-
- Grohskopf LA, Huskins WC, Sinkowitz-Cochran RL, Levine GL, Goldmann DA, Jarvis WR. Pediatr. Infect. Dis. J. 2005;24:766–773. - PubMed
-
- Twiss J, Byrnes C, Johnson R, Holland D. Int. J. Pharm. 2005;295:113–119. - PubMed
-
- Bacon JA, Linseman DA, Raczniak TJ. Toxicol. in Vitro. 1990;4:384–388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

