The construction of transgenic and gene knockout/knockin mouse models of human disease
- PMID: 21800101
- PMCID: PMC3516403
- DOI: 10.1007/s11248-011-9537-3
The construction of transgenic and gene knockout/knockin mouse models of human disease
Abstract
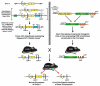
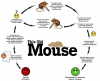
The genetic and physiological similarities between mice and humans have focused considerable attention on rodents as potential models of human health and disease. Together with the wealth of resources, knowledge, and technologies surrounding the mouse as a model system, these similarities have propelled this species to the forefront of biomedical research. The advent of genomic manipulation has quickly led to the creation and use of genetically engineered mice as powerful tools for cutting edge studies of human disease research including the discovery, refinement, and utility of many currently available therapeutic regimes. In particular, the creation of genetically modified mice as models of human disease has remarkably changed our ability to understand the molecular mechanisms and cellular pathways underlying disease states. Moreover, the mouse models resulting from gene transfer technologies have been important components correlating an individual's gene expression profile to the development of disease pathologies. The objective of this review is to provide physician-scientists with an expansive historical and logistical overview of the creation of mouse models of human disease through gene transfer technologies. Our expectation is that this will facilitate on-going disease research studies and may initiate new areas of translational research leading to enhanced patient care.
Figures





References
-
- Amanullah A, Liebermann DA, Hoffman B. Deregulated c-myc prematurely recruits both type i and ii cd95/fas apoptotic pathways associated with terminal myeloid differentiation. Oncogene. 2002;21:1600–1610. - PubMed
-
- Banting FG, Best CH. Pancreatic extracts.1922. J Lab Clin Med. 1990;115:254–272. - PubMed
-
- Bosma GC, Custer RP, Bosma MJ. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. - PubMed
-
- Branda CS, Dymecki SM. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Dev Cell. 2004;6:7–28. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

