Expression of vesicular glutamate transporters type 1 and 2 in sensory and autonomic neurons innervating the mouse colorectum
- PMID: 21800314
- PMCID: PMC3919020
- DOI: 10.1002/cne.22730
Expression of vesicular glutamate transporters type 1 and 2 in sensory and autonomic neurons innervating the mouse colorectum
Abstract
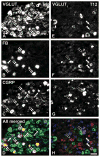
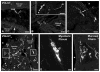
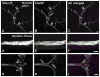
Vesicular glutamate transporters (VGLUTs) have been extensively studied in various neuronal systems, but their expression in visceral sensory and autonomic neurons remains to be analyzed in detail. Here we studied VGLUTs type 1 and 2 (VGLUT(1) and VGLUT(2) , respectively) in neurons innervating the mouse colorectum. Lumbosacral and thoracolumbar dorsal root ganglion (DRG), lumbar sympathetic chain (LSC), and major pelvic ganglion (MPG) neurons innervating the colorectum of BALB/C mice were retrogradely traced with Fast Blue, dissected, and processed for immunohistochemistry. Tissue from additional naïve mice was included. Previously characterized antibodies against VGLUT(1) , VGLUT(2) , and calcitonin gene-related peptide (CGRP) were used. Riboprobe in situ hybridization, using probes against VGLUT(1) and VGLUT(2) , was also performed. Most colorectal DRG neurons expressed VGLUT(2) and often colocalized with CGRP. A smaller percentage of neurons expressed VGLUT(1) . VGLUT(2) -immunoreactive (IR) neurons in the MPG were rare. Abundant VGLUT(2) -IR nerves were detected in all layers of the colorectum; VGLUT(1) -IR nerves were sparse. A subpopulation of myenteric plexus neurons expressed VGLUT2 protein and mRNA, but VGLUT1 mRNA was undetectable. In conclusion, we show 1) that most colorectal DRG neurons express VGLUT(2) , and to a lesser extent, VGLUT(1) ; 2) abundance of VGLUT2-IR fibers innervating colorectum; and 3) a subpopulation of myenteric plexus neurons expressing VGLUT(2). Altogether, our data suggests a role for VGLUT(2) in colorectal glutamatergic neurotransmission, potentially influencing colorectal sensitivity and motility.
Copyright © 2011 Wiley-Liss, Inc.
Figures






References
-
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. - PubMed
-
- Adams JC. Biotin amplification of biotin and horseradish peroxidase signals in histochemical stains. J Histochem Cytochem. 1992;40:1457–1463. - PubMed
-
- Aihara Y, Mashima H, Onda H, Hisano S, Kasuya H, Hori T, Yamada S, Tomura H, Yamada Y, Inoue I, Kojima I, Takeda J. Molecular cloning of a novel brain-type Na(+)-dependent inorganic phosphate cotransporter. J Neurochem. 2000;74:2622–2625. - PubMed
-
- Alvarez FJ, Villalba RM, Zerda R, Schneider SP. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol. 2004;472:257–280. - PubMed
-
- Anlauf E, Derouiche A. Astrocytic exocytosis vesicles and glutamate: a high-resolution immunofluorescence study. Glia. 2005;49:96–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

