Extracellular inhibitors, repellents, and semaphorin/plexin/MICAL-mediated actin filament disassembly
- PMID: 21800438
- PMCID: PMC3612987
- DOI: 10.1002/cm.20527
Extracellular inhibitors, repellents, and semaphorin/plexin/MICAL-mediated actin filament disassembly
Abstract
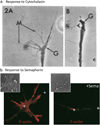
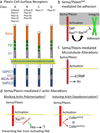
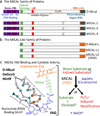
Multiple extracellular signals have been identified that regulate actin dynamics within motile cells, but how these instructive cues present on the cell surface exert their precise effects on the internal actin cytoskeleton is still poorly understood. One particularly interesting class of these cues is a group of extracellular proteins that negatively alter the movement of cells and their processes. Over the years, these types of events have been described using a variety of terms and herein we provide an overview of inhibitory/repulsive cellular phenomena and highlight the largest known protein family of repulsive extracellular cues, the Semaphorins. Specifically, the Semaphorins (Semas) utilize Plexin cell-surface receptors to dramatically collapse the actin cytoskeleton and we summarize what is known of the direct molecular and biochemical mechanisms of Sema-triggered actin filament (F-actin) disassembly. We also discuss new observations from our lab that reveal that the multidomain oxidoreductase (Redox) enzyme Molecule Interacting with CasL (MICAL), an important mediator of Sema/Plexin repulsion, is a novel F-actin disassembly factor. Our results indicate that MICAL triggers Sema/Plexin-mediated reorganization of the F-actin cytoskeleton and suggest a role for specific Redox signaling events in regulating actin dynamics.
Copyright © 2011 Wiley-Liss, Inc.
Figures




References
-
- Abercrombie M. Contact inhibition in tissue culture. In Vitro. 1970;6:128–142. - PubMed
-
- Abercrombie M. The crawling movement of metazoan cells. Proc R Soc Lond B Biol Sci. 1980;207(1167):129–147.
-
- Abercrombie M, Heaysman JE. Observations on the social behaviour of cells in tissue culture. I. Speed of movement of chick heart fibroblasts in relation to their mutual contacts. Exp Cell Res. 1953;5(1):111–131. - PubMed
-
- Aguayo AJ, Bray GM, Perkins CS, Duncan ID. Axon-sheath cell interactions in peripheral and central nervous system transplants. Society for neuroscience symposia: aspects of developmental neurobiology. 1979;4:361–383.
-
- Aizawa H, Wakatsuki S, Ishii A, Moriyama K, Sasaki Y, Ohashi K, Sekine-Aizawa Y, Sehara-Fujisawa A, Mizuno K, Goshima Y, et al. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A- induced growth cone collapse. Nat Neurosci. 2001;4(4):367–373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

