Regulation of chaperone/effector complex synthesis in a bacterial type III secretion system
- PMID: 21801239
- PMCID: PMC3909996
- DOI: 10.1111/j.1365-2958.2011.07784.x
Regulation of chaperone/effector complex synthesis in a bacterial type III secretion system
Abstract
Type III protein secretion systems (T3SSs), which have evolved to deliver bacterial proteins into nucleated cells, are found in many species of Gram-negative bacteria that live in close association with eukaryotic hosts. Proteins destined to travel this secretion pathway are targeted to the secretion machine by customized chaperones, with which they form highly structured complexes. Here, we have identified a mechanism that co-ordinates the expression of the Salmonella Typhimurium T3SS chaperone SicP and its cognate effector SptP. Translation of the effector is coupled to that of its chaperone, and in the absence of translational coupling, an inhibitory RNA structure prevents translation of sptP. The data presented here show how the genomic organization of functionally related proteins can have a significant impact on the co-ordination of their expression.
© 2011 Blackwell Publishing Ltd.
Figures

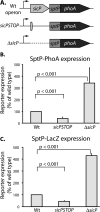
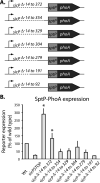

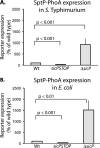
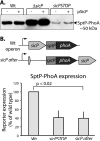

Comment in
-
Bacterial secretion: coupled translation of effector-chaperone partners.Nat Rev Microbiol. 2011 Sep 6;9(10):697. doi: 10.1038/nrmicro2657. Nat Rev Microbiol. 2011. PMID: 21894170 No abstract available.
References
-
- Blocker A, Jouihri N, Larquet E, Gounon P, Ebel F, Parsot C, Sansonetti P, Allaoui A. Structure and composition of the Shigella flexneri “needle complex”, a part of its type III secreton. Molecular microbiology. 2001;39:652–663. - PubMed
-
- Cornelis G. The type III secretion injectisome. Nat Rev Microbiol. 2006;4:811–825. - PubMed
-
- Demerec M, Hartman PE. Complex loci in microorganisms. Annual Review of Microbiology. 1959;13:377–406.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

