In vitro analyses of the anti-fibrotic effect of SPARC silencing in human Tenon's fibroblasts: comparisons with mitomycin C
- PMID: 21801304
- PMCID: PMC3823078
- DOI: 10.1111/j.1582-4934.2011.01400.x
In vitro analyses of the anti-fibrotic effect of SPARC silencing in human Tenon's fibroblasts: comparisons with mitomycin C
Abstract
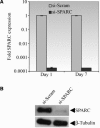
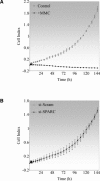
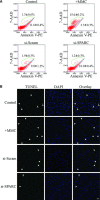
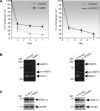
Failure of glaucoma filtration surgery (GFS) is commonly attributed to scarring at the surgical site. The human Tenon's fibroblasts (HTFs) are considered the major cell type contributing to the fibrotic response. We previously showed that SPARC (secreted protein, acidic, rich in cysteine) knockout mice had improved surgical success in a murine model of GFS. To understand the mechanisms of SPARC deficiency in delaying subconjunctival fibrosis, we used the gene silencing approach to reduce SPARC expression in HTFs and examined parameters important for wound repair and fibrosis. Mitomycin C-treated HTFs were used for comparison. We demonstrate that SPARC-silenced HTFs showed normal proliferation and negligible cellular necrosis but were impaired in motility and collagen gel contraction. The expression of pro-fibrotic genes including collagen I, MMP-2, MMP-9, MMP-14, IL-8, MCP-1 and TGF-β(2) were also reduced. Importantly, TGF-β(2) failed to induce significant collagen I and fibronectin expressions in the SPARC-silenced HTFs. Together, these data demonstrate that SPARC knockdown in HTFs modulates fibroblast functions important for wound fibrosis and is therefore a promising strategy in the development of anti-scarring therapeutics.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures



Similar articles
-
SPARC is expressed in scars of the Tenon's capsule and mediates scarring properties of human Tenon's fibroblasts in vitro.Mol Vis. 2011 Jan 19;17:177-85. Mol Vis. 2011. PMID: 21264231 Free PMC article.
-
SPARC-YAP/TAZ inhibition prevents the fibroblasts-myofibroblasts transformation.Exp Cell Res. 2023 Aug 1;429(1):113649. doi: 10.1016/j.yexcr.2023.113649. Epub 2023 May 22. Exp Cell Res. 2023. PMID: 37225012
-
Effects of atorvastatin on the function of Tenon's capsule fibroblasts in human eyes.Int Ophthalmol. 2023 Oct;43(10):3707-3715. doi: 10.1007/s10792-023-02780-5. Epub 2023 Jul 8. Int Ophthalmol. 2023. PMID: 37422546
-
The role of secreted protein acidic and rich in cysteine (SPARC) in cardiac repair and fibrosis: Does expression of SPARC by macrophages influence outcomes?J Mol Cell Cardiol. 2016 Apr;93:156-61. doi: 10.1016/j.yjmcc.2015.11.014. Epub 2015 Nov 12. J Mol Cell Cardiol. 2016. PMID: 26582465 Review.
-
Developing novel anti-fibrotic therapeutics to modulate post-surgical wound healing in glaucoma: big potential for small molecules.Expert Rev Ophthalmol. 2015 Feb;10(1):65-76. doi: 10.1586/17469899.2015.983475. Expert Rev Ophthalmol. 2015. PMID: 25983855 Free PMC article. Review.
Cited by
-
SPARC: a potential target for functional nanomaterials and drugs.Front Mol Biosci. 2023 Jul 28;10:1235428. doi: 10.3389/fmolb.2023.1235428. eCollection 2023. Front Mol Biosci. 2023. PMID: 37577749 Free PMC article. Review.
-
Upregulation of distinct collagen transcripts in post-surgery scar tissue: a study of conjunctival fibrosis.Dis Model Mech. 2017 Jun 1;10(6):751-760. doi: 10.1242/dmm.028555. Epub 2017 Mar 22. Dis Model Mech. 2017. PMID: 28331057 Free PMC article.
-
Novel Therapies for the Prevention of Fibrosis in Glaucoma Filtration Surgery.Biomedicines. 2023 Feb 21;11(3):657. doi: 10.3390/biomedicines11030657. Biomedicines. 2023. PMID: 36979636 Free PMC article. Review.
-
Matrix Metalloproteinases and Glaucoma.Biomolecules. 2022 Sep 25;12(10):1368. doi: 10.3390/biom12101368. Biomolecules. 2022. PMID: 36291577 Free PMC article. Review.
-
Transcriptional dynamics of a conserved gene expression network associated with craniofacial divergence in Arctic charr.Evodevo. 2014 Nov 3;5(1):40. doi: 10.1186/2041-9139-5-40. eCollection 2014. Evodevo. 2014. PMID: 25419450 Free PMC article.
References
-
- Heijl A, Leske MC, Bengtsson B. Reduction of intraocular pressure and glaucoma progression: results from the early manifest glaucoma trial. Arch Ophthalmol. 2002;120:1268–78. - PubMed
-
- Kass MA, Heuer DK, Higginbotham EJ, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13. - PubMed
-
- Hitchings RA, Grierson I. Clinico pathological correlation in eyes with failed fistulizing surgery. Trans Ophthalmol Soc UK. 1983;103:84–8. - PubMed
-
- Skuta GL, Parrish RK. Wound healing in glaucoma filtering surgery. Surv Ophthalmol. 1987;32:149–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

