Matrix metalloproteinase-1 contribution to sarcoma cell invasion
- PMID: 21801306
- PMCID: PMC3823085
- DOI: 10.1111/j.1582-4934.2011.01402.x
Matrix metalloproteinase-1 contribution to sarcoma cell invasion
Abstract
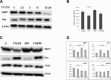
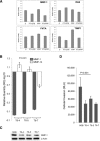
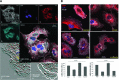
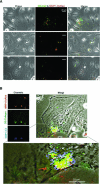
Matrix metalloproteinase-1 (MMP-1) activity has been linked to numerous disease processes from arthritis to ulcer. Its proteolytic activity has been implicated inconsistently in different steps of tumourigenesis and metastasis. The discrepancies may be attributable to our limited understanding of MMP-1 production, cellular trafficking, secretion and local activation. Specifically, regulation of MMP-1 directional delivery versus its general extracellular matrix secretion is largely unknown. Inhibition of prenylation by farnesyl transferase inhibitor (FTI-276) decreased extracellular MMP-1 and subsequently reduced invasiveness by 30%. Parallel, stable cell line RNAi knockdown of MMP-1 confirmed its role in cellular invasiveness. The prenylation agonist farnesyl pyrophosphate (FPP) partially normalized FTI-276 inhibited extracellular MMP-1 levels and invasion capacity while transiently delayed its cellular podia distribution. MMP-1 directional delivery to these structures were confirmed by combination of a MMP-1-specific fluorogenic substrate, a MMP1-Ds-Red fusion protein construct expression and DQ-collagen degradation, which demonstrated coupling of directional delivery and activation. MetaMorph analysis of cellular lamellipodia structures indicated that FTI-276 inhibited formation and delivery to these structures. Farnesyl pyrophosphate partially restored lamellipodia area but not MMP-1 delivery under the time frame investigated. These results indicate that MMP-1 directional delivery to podia structures is involved in the invasive activity of sarcoma cells, and this process is prenylation sensitive.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures

Similar articles
-
Protein isoprenylation regulates secretion of matrix metalloproteinase 1 from rheumatoid synovial fibroblasts: effects of statins and farnesyl and geranylgeranyl transferase inhibitors.Arthritis Rheum. 2007 Sep;56(9):2840-53. doi: 10.1002/art.22824. Arthritis Rheum. 2007. PMID: 17763406
-
Increased expression of matrix metalloproteinases mediates thromboxane A2-induced invasion in lung cancer cells.Curr Cancer Drug Targets. 2012 Jul;12(6):703-15. doi: 10.2174/156800912801784884. Curr Cancer Drug Targets. 2012. PMID: 22515524
-
Roles of membrane type 1 matrix metalloproteinase and tissue inhibitor of metalloproteinases 2 in invasion and dissemination of human malignant glioma.J Neurosurg. 2001 Mar;94(3):464-73. doi: 10.3171/jns.2001.94.3.0464. J Neurosurg. 2001. PMID: 11235952
-
Specialized surface protrusions of invasive cells, invadopodia and lamellipodia, have differential MT1-MMP, MMP-2, and TIMP-2 localization.Ann N Y Acad Sci. 1999 Jun 30;878:361-71. doi: 10.1111/j.1749-6632.1999.tb07695.x. Ann N Y Acad Sci. 1999. PMID: 10415741 Review.
-
Membrane-type 1 matrix metalloproteinase: a key enzyme for tumor invasion.Cancer Lett. 2003 May 8;194(1):1-11. doi: 10.1016/s0304-3835(02)00699-7. Cancer Lett. 2003. PMID: 12706853 Review.
Cited by
-
New tricks for human farnesyltransferase inhibitor: cancer and beyond.Medchemcomm. 2017 Feb 16;8(5):841-854. doi: 10.1039/c7md00030h. eCollection 2017 May 1. Medchemcomm. 2017. PMID: 30108801 Free PMC article. Review.
-
An novel role of sphingosine kinase-1 (SPHK1) in the invasion and metastasis of esophageal carcinoma.J Transl Med. 2011 Sep 22;9:157. doi: 10.1186/1479-5876-9-157. J Transl Med. 2011. PMID: 21936950 Free PMC article.
-
Direct measurement of matrix metalloproteinase activity in 3D cellular microenvironments using a fluorogenic peptide substrate.Biomaterials. 2013 Oct;34(30):7344-52. doi: 10.1016/j.biomaterials.2013.06.023. Epub 2013 Jul 2. Biomaterials. 2013. PMID: 23830581 Free PMC article.
-
The roles of beta-adrenergic receptors in tumorigenesis and the possible use of beta-adrenergic blockers for cancer treatment: possible genetic and cell-signaling mechanisms.Cancer Manag Res. 2012;4:431-45. doi: 10.2147/CMAR.S39153. Epub 2012 Dec 18. Cancer Manag Res. 2012. PMID: 23293538 Free PMC article.
-
Assessing the carcinogenic potential of low-dose exposures to chemical mixtures in the environment: focus on the cancer hallmark of tumor angiogenesis.Carcinogenesis. 2015 Jun;36 Suppl 1(Suppl 1):S184-202. doi: 10.1093/carcin/bgv036. Carcinogenesis. 2015. PMID: 26106137 Free PMC article. Review.
References
-
- Jiang X, Dutton CM, Qi WN, et al. siRNA mediated inhibition of MMP-1 reduces invasive potential of a human chondrosarcoma cell line. J Cell Physiol. 2005;202:723–30. - PubMed
-
- Pei D. Matrix metalloproteinases target protease-activated receptors on the tumour cell surface. Cancer Cell. 2005;7:207–8. - PubMed
-
- Polette M, Clavel C, Cockett M, et al. Detection and localization of mRNAs encoding matrix metalloproteinases and their tissue inhibitor in human breast pathology. Invasion Metast. 1993;13:31–7. - PubMed
-
- Pritchard SC, Nicolson MC, Lloret C, et al. Expression of matrix metalloproteinases 1, 2, 9 and their tissue inhibitors in stage II non-small cell lung cancer: implications for MMP inhibition therapy. Oncol Rep. 2001;8:421–4. - PubMed
-
- Vachani A, Nebozhyn M, Singhal S, et al. A 10-gene classifier for distinguishing head and neck squamous cell carcinoma and lung squamous cell carcinoma. Clin Cancer Res. 2007;13:2905–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

