Chikungunya virus adaptation to Aedes albopictus mosquitoes does not correlate with acquisition of cholesterol dependence or decreased pH threshold for fusion reaction
- PMID: 21801412
- PMCID: PMC3162544
- DOI: 10.1186/1743-422X-8-376
Chikungunya virus adaptation to Aedes albopictus mosquitoes does not correlate with acquisition of cholesterol dependence or decreased pH threshold for fusion reaction
Abstract
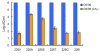
Background: Chikungunya virus (CHIKV) is a mosquito transmitted alphavirus that recently caused several large scale outbreaks/epidemics of arthritic disease in tropics of Africa, Indian Ocean basin and South-East Asia. This re-emergence event was facilitated by genetic adaptation (E1-A226V substitution) of CHIKV to a newly significant mosquito vector for this virus; Aedes albopictus. However, the molecular mechanism explaining the positive effect of the E1-A226V mutation on CHIKV fitness in this vector remains largely unknown. Previously we demonstrated that the E1-A226V substitution is also associated with attenuated CHIKV growth in cells depleted by cholesterol.
Methods: In this study, using a panel of CHIKV clones that varies in sensitivity to cholesterol, we investigated the possible relationship between cholesterol dependence and Ae. albopictus infectivity.
Results: We demonstrated that there is no clear mechanistic correlation between these two phenotypes. We also showed that the E1-A226V mutation increases the pH dependence of the CHIKV fusion reaction; however, subsequent genetic analysis failed to support an association between CHIKV dependency on lower pH, and mosquito infectivity phenotypes.
Conclusion: the E1-A226V mutation probably acts at different steps of the CHIKV life cycle, affecting multiple functions of the virus.
Figures

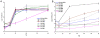





Similar articles
-
pH-dependent entry of chikungunya virus into Aedes albopictus cells.Infect Genet Evol. 2012 Aug;12(6):1275-81. doi: 10.1016/j.meegid.2012.02.003. Epub 2012 Feb 20. Infect Genet Evol. 2012. PMID: 22386853
-
Epistatic roles of E2 glycoprotein mutations in adaption of chikungunya virus to Aedes albopictus and Ae. aegypti mosquitoes.PLoS One. 2009 Aug 31;4(8):e6835. doi: 10.1371/journal.pone.0006835. PLoS One. 2009. PMID: 19718263 Free PMC article.
-
Vertical transmission of Indian Ocean Lineage of chikungunya virus in Aedes aegypti and Aedes albopictus mosquitoes.Parasit Vectors. 2016 Apr 23;9:227. doi: 10.1186/s13071-016-1505-6. Parasit Vectors. 2016. PMID: 27108077 Free PMC article.
-
Chikungunya virus and its mosquito vectors.Vector Borne Zoonotic Dis. 2015 Apr;15(4):231-40. doi: 10.1089/vbz.2014.1745. Epub 2015 Feb 12. Vector Borne Zoonotic Dis. 2015. PMID: 25674945 Review.
-
Chikungunya virus infection: molecular biology, clinical characteristics, and epidemiology in Asian countries.J Biomed Sci. 2021 Dec 2;28(1):84. doi: 10.1186/s12929-021-00778-8. J Biomed Sci. 2021. PMID: 34857000 Free PMC article. Review.
Cited by
-
Emerging Chikungunya Virus Variants at the E1-E1 Interglycoprotein Spike Interface Impact Virus Attachment and Inflammation.J Virol. 2022 Feb 23;96(4):e0158621. doi: 10.1128/JVI.01586-21. Epub 2021 Dec 22. J Virol. 2022. PMID: 34935436 Free PMC article.
-
Chikungunya virus glycoproteins pseudotype with lentiviral vectors and reveal a broad spectrum of cellular tropism.PLoS One. 2014 Oct 21;9(10):e110893. doi: 10.1371/journal.pone.0110893. eCollection 2014. PLoS One. 2014. PMID: 25333782 Free PMC article.
-
Evolution-Driven Attenuation of Alphaviruses Highlights Key Glycoprotein Determinants Regulating Viral Infectivity and Dissemination.Cell Rep. 2019 Jul 9;28(2):460-471.e5. doi: 10.1016/j.celrep.2019.06.022. Cell Rep. 2019. PMID: 31291581 Free PMC article.
-
Factors Affecting Arbovirus Midgut Escape in Mosquitoes.Pathogens. 2023 Jan 31;12(2):220. doi: 10.3390/pathogens12020220. Pathogens. 2023. PMID: 36839492 Free PMC article. Review.
-
Chikungunya virus infection of cell lines: analysis of the East, Central and South African lineage.PLoS One. 2012;7(1):e31102. doi: 10.1371/journal.pone.0031102. Epub 2012 Jan 27. PLoS One. 2012. PMID: 22299053 Free PMC article.
References
-
- Delatte H, Paupy C, Dehecq JS, Thiria J, Failloux AB, Fontenille D. [Aedes albopictus, vector of chikungunya and dengue viruses in Reunion Island: biology and control] Parasite. 2008;15:3–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

