Alternative antiretroviral monitoring strategies for HIV-infected patients in east Africa: opportunities to save more lives?
- PMID: 21801434
- PMCID: PMC3163507
- DOI: 10.1186/1758-2652-14-38
Alternative antiretroviral monitoring strategies for HIV-infected patients in east Africa: opportunities to save more lives?
Abstract
Background: Updated World Health Organization guidelines have amplified debate about how resource constraints should impact monitoring strategies for HIV-infected persons on combination antiretroviral therapy (cART). We estimated the incremental benefit and cost effectiveness of alternative monitoring strategies for east Africans with known HIV infection.
Methods: Using a validated HIV computer simulation based on resource-limited data (USAID and AMPATH) and circumstances (east Africa), we compared alternative monitoring strategies for HIV-infected persons newly started on cART. We evaluated clinical, immunologic and virologic monitoring strategies, including combinations and conditional logic (e.g., only perform virologic testing if immunologic testing is positive). We calculated incremental cost-effectiveness ratios (ICER) in units of cost per quality-adjusted life year (QALY), using a societal perspective and a lifetime horizon. Costs were measured in 2008 US dollars, and costs and benefits were discounted at 3%. We compared the ICER of monitoring strategies with those of other resource-constrained decisions, in particular earlier cART initiation (at CD4 counts of 350 cells/mm3 rather than 200 cells/mm3).
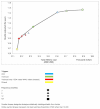
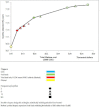
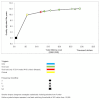
Results: Monitoring strategies employing routine CD4 testing without virologic testing never maximized health benefits, regardless of budget or societal willingness to pay for additional health benefits. Monitoring strategies employing virologic testing conditional upon particular CD4 results delivered the most benefit at willingness-to-pay levels similar to the cost of earlier cART initiation (approximately $2600/QALY). Monitoring strategies employing routine virologic testing alone only maximized health benefits at willingness-to-pay levels (> $4400/QALY) that greatly exceeded the ICER of earlier cART initiation.
Conclusions: CD4 testing alone never maximized health benefits regardless of resource limitations. Programmes routinely performing virologic testing but deferring cART initiation may increase health benefits by reallocating monitoring resources towards earlier cART initiation.
Figures

References
-
- Elliott JH, Lynen L, Calmy A, De Luca A, Shafer RW, Zolfo M, Clotet B, Huffam S, Boucher CA, Cooper DA, Schapiro JM. Rational use of antiretroviral therapy in low-income and middle-income countries: optimizing regimen sequencing and switching. AIDS. 2008;14:2053–2067. doi: 10.1097/QAD.0b013e328309520d. - DOI - PubMed
-
- Johannessen A, Garrido C, Zahonero N, Sandvik L, Naman E, Kivuyo SL, Kasubi MJ, Gundersen SG, Bruun JN, de Mendoza C. Dried blood spots perform well in viral load monitoring of patients who receive antiretroviral treatment in rural Tanzania. Clin Infect Dis. 2009;14:976–981. doi: 10.1086/605502. - DOI - PubMed
-
- Phillips AN, Pillay D, Miners AH, Bennett DE, Gilks CF, Lundgren JD. Outcomes from monitoring of patients on antiretroviral therapy in resource-limited settings with viral load, CD4 cell count, or clinical observation alone: a computer simulation model. Lancet. 2008;14:1443–1451. doi: 10.1016/S0140-6736(08)60624-8. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

