Seroprevalence of trichodysplasia spinulosa-associated polyomavirus
- PMID: 21801610
- PMCID: PMC3381547
- DOI: 10.3201/eid1708.110114
Seroprevalence of trichodysplasia spinulosa-associated polyomavirus
Abstract
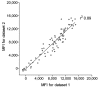
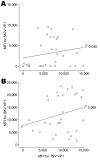
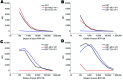
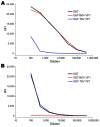
We identified a new polyomavirus in skin lesions from a patient with trichodysplasia spinulosa (TS). Apart from TS being an extremely rare disease, little is known of its epidemiology. On the basis of knowledge regarding other polyomaviruses, we anticipated that infections with trichodysplasia spinulosa-associated polyomavirus (TSV) occur frequently and become symptomatic only in immunocompromised patients. To investigate this hypothesis, we developed and used a Luminex-based TSV viral protein 1 immunoassay, excluded cross-reactivity with phylogenetically related Merkel cell polyomavirus, and measured TSV seroreactivity. Highest reactivity was found in a TS patient. In 528 healthy persons in the Netherlands, a wide range of seroreactivities was measured and resulted in an overall TSV seroprevalence of 70% (range 10% in small children to 80% in adults). In 80 renal transplant patients, seroprevalence was 89%. Infection with the new TSV polyomavirus is common and occurs primarily at a young age.
Figures



Similar articles
-
Seroepidemiology of the newly found trichodysplasia spinulosa-associated polyomavirus.J Infect Dis. 2011 Nov 15;204(10):1523-6. doi: 10.1093/infdis/jir614. Epub 2011 Sep 16. J Infect Dis. 2011. PMID: 21926381
-
Seroprevalence of trichodysplasia spinulosa-associated polyomavirus in Japan.J Clin Virol. 2015 Apr;65:76-82. doi: 10.1016/j.jcv.2015.02.014. Epub 2015 Feb 20. J Clin Virol. 2015. PMID: 25766994
-
Trichodysplasia spinulosa-associated polyomavirus (TSV) and Merkel cell polyomavirus: correlation between humoral and cellular immunity stronger with TSV.PLoS One. 2012;7(9):e45773. doi: 10.1371/journal.pone.0045773. Epub 2012 Sep 24. PLoS One. 2012. PMID: 23029236 Free PMC article.
-
[New, newer, newest human polyomaviruses: how far?].Mikrobiyol Bul. 2013 Apr;47(2):362-81. doi: 10.5578/mb.5377. Mikrobiyol Bul. 2013. PMID: 23621738 Review. Turkish.
-
Human polyomaviruses in disease and cancer.Virology. 2013 Mar 15;437(2):63-72. doi: 10.1016/j.virol.2012.12.015. Epub 2013 Jan 26. Virology. 2013. PMID: 23357733 Review.
Cited by
-
Age-specific seroprevalences of merkel cell polyomavirus, human polyomaviruses 6, 7, and 9, and trichodysplasia spinulosa-associated polyomavirus.Clin Vaccine Immunol. 2013 Mar;20(3):363-8. doi: 10.1128/CVI.00438-12. Epub 2013 Jan 9. Clin Vaccine Immunol. 2013. PMID: 23302741 Free PMC article.
-
Polyomaviruses and disease: is there more to know than viremia and viruria?Curr Opin Organ Transplant. 2015 Jun;20(3):348-58. doi: 10.1097/MOT.0000000000000192. Curr Opin Organ Transplant. 2015. PMID: 25933251 Free PMC article. Review.
-
Trichodysplasia spinulosa-Associated Polyomavirus Uses a Displaced Binding Site on VP1 to Engage Sialylated Glycolipids.PLoS Pathog. 2015 Aug 24;11(8):e1005112. doi: 10.1371/journal.ppat.1005112. eCollection 2015 Aug. PLoS Pathog. 2015. PMID: 26302170 Free PMC article.
-
Seroprevalence and cross-reactivity of human polyomavirus 9.Emerg Infect Dis. 2012 Aug;18(8):1329-32. doi: 10.3201/eid1808.111625. Emerg Infect Dis. 2012. PMID: 22840602 Free PMC article.
-
Seroprevalence of Four Polyomaviruses Linked to Dermatological Diseases: New Findings and a Comprehensive Analysis.Viruses. 2022 Oct 17;14(10):2282. doi: 10.3390/v14102282. Viruses. 2022. PMID: 36298837 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
