Regulation of HMG-CoA reductase in mammals and yeast
- PMID: 21801748
- PMCID: PMC3184313
- DOI: 10.1016/j.plipres.2011.07.002
Regulation of HMG-CoA reductase in mammals and yeast
Abstract
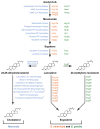
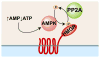
HMG-CoA reductase (HMGR), a highly conserved, membrane-bound enzyme, catalyzes a rate-limiting step in sterol and isoprenoid biosynthesis and is the primary target of hypocholesterolemic drug therapy. HMGR activity is tightly regulated to ensure maintenance of lipid homeostasis, disruption of which is a major cause of human morbidity and mortality. HMGR regulation takes place at the levels of transcription, translation, post-translational modification and degradation. In this review, we discuss regulation of mammalian, Saccharomyces cerevisiae and Schizosaccharomyces pombe HMGR and highlight recent advances in the field. We find that the general features of HMGR regulation, including a requirement for the HMGR-binding protein Insig, are remarkably conserved between mammals and ascomycetous fungi, including S. cerevisiae and S. pombe. However the specific details by which this regulation occurs differ in surprising ways, revealing the broad evolutionary themes underlying both HMGR regulation and Insig function.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures




References
-
- Goldstein JL. A Receptor-mediated pathway for cholesterol homeostasis. In: Frängsmyr T, Lindsten J, editors. Nobel Lectures, Physiology or Medicine 1981–1990. Singapore: World Scientific Publishing Co; 1993.
-
- Yeagle PL. Lipid regulation of cell membrane structure and function. FASEB J. 1989;3:1833–42. - PubMed
-
- Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274:255–9. - PubMed
-
- Vance DE, Van den Bosch H. Cholesterol in the year 2000. Biochim Biophys Acta. 2000;1529:1–8. - PubMed
-
- Andreasen AA, Stier TJB. Anaerobic nutrition of Saccharomyces cerevisiae. I. Ergosterol requirement for growth in a defined medium. J Cell Physiol. 1953;41:23–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

