Semaphorin-PlexinD1 signaling limits angiogenic potential via the VEGF decoy receptor sFlt1
- PMID: 21802375
- PMCID: PMC3156278
- DOI: 10.1016/j.devcel.2011.06.033
Semaphorin-PlexinD1 signaling limits angiogenic potential via the VEGF decoy receptor sFlt1
Abstract
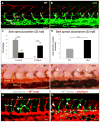
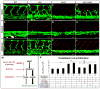
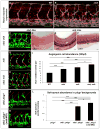
Sprouting angiogenesis expands the embryonic vasculature enabling survival and homeostasis. Yet how the angiogenic capacity to form sprouts is allocated among endothelial cells (ECs) to guarantee the reproducible anatomy of stereotypical vascular beds remains unclear. Here we show that Sema-PlxnD1 signaling, previously implicated in sprout guidance, represses angiogenic potential to ensure the proper abundance and stereotypical distribution of the trunk's segmental arteries (SeAs). We find that Sema-PlxnD1 signaling exerts this effect by antagonizing the proangiogenic activity of vascular endothelial growth factor (VEGF). Specifically, Sema-PlxnD1 signaling ensures the proper endothelial abundance of soluble flt1 (sflt1), an alternatively spliced form of the VEGF receptor Flt1 encoding a potent secreted decoy. Hence, Sema-PlxnD1 signaling regulates distinct but related aspects of angiogenesis: the spatial allocation of angiogenic capacity within a primary vessel and sprout guidance.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Comment in
-
Semaphorin signals on the road of endothelial tip cells.Dev Cell. 2011 Aug 16;21(2):189-90. doi: 10.1016/j.devcel.2011.07.017. Dev Cell. 2011. PMID: 21839915
References
-
- Acevedo LM, Cheresh DA. Suppressing NFAT increases VEGF signaling in hemangiomas. Cancer Cell. 2008;14:429–430. - PubMed
-
- Ahn DG, Ruvinsky I, Oates AC, Silver LM, Ho RK. tbx20, a new vertebrate T-box gene expressed in the cranial motor neurons and developing cardiovascular structures in zebrafish. Mech Dev. 2000;95:253–258. - PubMed
-
- Aref S, El Sherbiny M, Goda T, Fouda M, Al Askalany H, Abdalla D. Soluble VEGF/sFLt1 ratio is an independent predictor of AML patient out come. Hematology. 2005;10:131–134. - PubMed
-
- Banu N, Teichman J, Dunlap-Brown M, Villegas G, Tufro A. Semaphorin 3C regulates endothelial cell function by increasing integrin activity. FASEB J. 2006;20:2150–2152. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

