Robotic catheter ablation of left ventricular tachycardia: initial experience
- PMID: 21802391
- PMCID: PMC3298677
- DOI: 10.1016/j.hrthm.2011.07.032
Robotic catheter ablation of left ventricular tachycardia: initial experience
Abstract
Background: Catheter ablation of ventricular tachycardia (VT) can be technically challenging due to difficulty with catheter positioning in the left ventricle (LV) and achieving stable contact. The Hansen Sensei Robotic system (HRS) has been used in atrial fibrillation but its utility in VT is unclear.
Objective: The purpose of this study was to test the technical feasibility of robotic catheter ablation of LV ventricular tachycardia (VT) using the HRS.
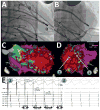
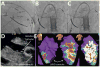
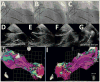
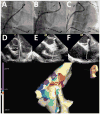
Methods: Twenty-three patients underwent LV VT mapping and ablation with the HRS via a transseptal, transmitral valve approach. Nineteen patients underwent substrate mapping and ablation (18 had ischemic cardiomyopathy, 1 had an apical variant of hypertrophic cardiomyopathy). Four patients had focal VT requiring LV VT mapping and ablation. Procedural endpoints included substrate modification by endocardial scar border ablation and elimination of late potentials, or elimination of inducible focal VT.
Results: Mapping and ablation were entirely robotic without requiring manual catheter manipulation in all patients and reaching all LV regions with stable contact. Fluoroscopy time of the LV procedure was 22.2 ± 11.2 minutes. Radiofrequency time was 33 ± 21 minutes. Total procedural times were 231 ± 76 minutes. Complications included a left groin hematoma (opposite to the HRS sheath), 1 pericardial effusion without tamponade that was drained successfully, and transient right ventricular failure in a patient with previous left ventricular assist device. At 13.4 ± 6.7 months of follow-up (range 1-19 months), recurrence of VT occurred in 3 of 23 patients.
Conclusion: Our initial experience suggests that the HRS allows successful mapping and ablation of LV VT.
Copyright © 2011 Heart Rhythm Society. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Stevenson WG, Wilber DJ, Natale A, et al. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: the multicenter thermocool ventricular tachycardia ablation trial. Circulation. 2008;118:2773–2782. - PubMed
-
- Kuck KH, Schaumann A, Eckardt L, et al. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet. 2010;375:31–40. - PubMed
-
- Aliot EM, Stevenson WG, Almendral-Garrote JM, et al. EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA) Heart Rhythm. 2009;6:886–933. - PubMed
-
- Wazni OM, Barrett C, Martin DO, et al. Experience with the hansen robotic system for atrial fibrillation ablation--lessons learned and techniques modified: Hansen in the real world. J Cardiovasc Electrophysiol. 2009;20:1193–1196. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

