Cytochrome c oxidase: evolution of control via nuclear subunit addition
- PMID: 21802404
- PMCID: PMC3923406
- DOI: 10.1016/j.bbabio.2011.07.007
Cytochrome c oxidase: evolution of control via nuclear subunit addition
Abstract
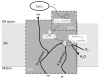
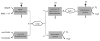
According to theory, present eukaryotic cells originated from a beneficial association between two free-living cells. Due to this endosymbiotic event the pre-eukaryotic cell gained access to oxidative phosphorylation (OXPHOS), which produces more than 15 times as much ATP as glycolysis. Because cellular ATP needs fluctuate and OXPHOS both requires and produces entities that can be toxic for eukaryotic cells such as ROS or NADH, we propose that the success of endosymbiosis has largely depended on the regulation of endosymbiont OXPHOS. Several studies have presented cytochrome c oxidase as a key regulator of OXPHOS; for example, COX is the only complex of mammalian OXPHOS with known tissue-specific isoforms of nuclear encoded subunits. We here discuss current knowledge about the origin of nuclear encoded subunits and the appearance of different isozymes promoted by tissue and cellular environments such as hypoxia. We also review evidence for recent selective pressure acting on COX among vertebrates, particularly in primate lineages, and discuss the unique pattern of co-evolution between the nuclear and mitochondrial genomes. Finally, even though the addition of nuclear encoded subunits was a major event in eukaryotic COX evolution, this does not lead to emergence of a more efficient COX, as might be expected from an anthropocentric point of view, for the "higher" organism possessing large brains and muscles. The main function of these subunits appears to be "only" to control the activity of the mitochondrial subunits. We propose that this control function is an as yet under appreciated key point of evolution. Moreover, the importance of regulating energy supply may have caused the addition of subunits encoded by the nucleus in a process comparable to a "domestication scenario" such that the host tends to control more and more tightly the ancestral activity of COX performed by the mtDNA encoded subunits.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures

References
-
- Richards TA, Archibald JM. Cell evolution: gene transfer agents and the origin of mitochondria. Curr Biol. 2011;21:R112–R114. - PubMed
-
- Zimmer C. Origins. On the origin of eukaryotes. Science. 2009;325:666–668. - PubMed
-
- Sagan L. On the origin of mitosing cells. J Theor Biol. 1967;14:255–274. - PubMed
-
- Veech RL. The metabolism of lactate. NMR Biomed. 1991;4:53–58. - PubMed
-
- Avery SV. Molecular targets of oxidative stress. Biochem J. 2011;434:201–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

