Hematopoietic stem and progenitor cell trafficking
- PMID: 21802990
- PMCID: PMC3185129
- DOI: 10.1016/j.it.2011.06.011
Hematopoietic stem and progenitor cell trafficking
Abstract
Migration of hematopoietic stem cells (HSCs) is essential during embryonic development and throughout adult life. During embryogenesis, trafficking of HSCs is responsible for the sequential colonization of different hematopoietic organs by blood-producing cells. In adulthood, circulation of HSCs maintains homeostasis of the hematopoietic system and participates in innate immune responses. HSC trafficking is also crucial in clinical settings such as bone marrow (BM) and stem cell transplantation. This review provides an overview of the molecular and cellular signals that control and fine-tune trafficking of HSCs and hematopoietic progenitor cells in embryogenesis and during postnatal life. We also discuss the potential clinical utility of therapeutic approaches to modulate HSC trafficking in patients.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
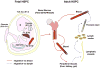
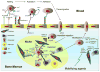
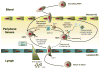
References
-
- Shizuru JA, et al. Hematopoietic stem and progenitor cells: clinical and preclinical regeneration of the hematolymphoid system. Annu Rev Med. 2005;56:509–538. - PubMed
-
- Muller-Sieburg CE, et al. Isolation of two early B lymphocyte progenitors from mouse marrow: a committed pre-pre-B cell and a clonogenic Thy-1-lo hematopoietic stem cell. Cell. 1986;44 (4):653–662. - PubMed
-
- Mempel T. In vivo imaging of leukocyte trafficking in blood vessels and tissues. Current Opinion in Immunology. 2004;16 (4):406–417. - PubMed
-
- Sumen C, et al. Intravital microscopy; visualizing immunity in context. Immunity. 2004;21 (3):315–329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

