Growth differentiation factor 15 (GDF15)-mediated HER2 phosphorylation reduces trastuzumab sensitivity of HER2-overexpressing breast cancer cells
- PMID: 21803025
- PMCID: PMC3191232
- DOI: 10.1016/j.bcp.2011.07.082
Growth differentiation factor 15 (GDF15)-mediated HER2 phosphorylation reduces trastuzumab sensitivity of HER2-overexpressing breast cancer cells
Abstract
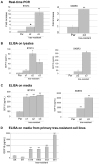
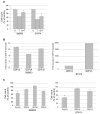
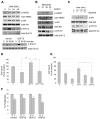
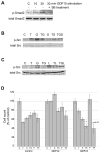
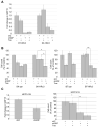
Resistance to the anti-HER2 monoclonal antibody trastuzumab is a major problem in the treatment of HER2-overexpressing metastatic breast cancer. Growth differentiation factor 15 (GDF15), which is structurally similar to TGF beta, has been reported to stimulate phosphorylation of HER2. We tested the hypothesis that GDF15-mediated phosphorylation of HER2 reduces the sensitivity of HER2-overexpressing breast cancer cell lines to trastuzumab. Gene microarray analysis, real-time PCR, and ELISA were used to assess GDF15 expression. Growth inhibition and proliferation assays in response to pharmacologic inhibitors of HER2, TGF beta receptor, or Src were performed on cells stimulated with recombinant human GDF15 or stable GDF15 transfectants. Western blotting was performed to determine effects of GDF15 on HER2 signaling. Cells were infected with lentiviral GDF15 shRNA plasmid to determine effects of GDF15 knockdown on cell survival in response to trastuzumab. Cells with acquired or primary trastuzumab resistance showed increased GDF15 expression. Exposure of trastuzumab-sensitive cells to recombinant human GDF15 or stable transfection of a GDF15 expression plasmid inhibited trastuzumab-mediated growth inhibition. HER2 tyrosine kinase inhibition abrogated GDF15-mediated Akt and Erk1/2 phosphorylation and blocked GDF15-mediated trastuzumab resistance. Pharmacologic inhibition of TGF beta receptor blocked GDF15-mediated phosphorylation of Src. Further, TGF beta receptor inhibition or Src inhibition blocked GDF15-mediated trastuzumab resistance. Finally, lentiviral GDF15 shRNA increased trastuzumab sensitivity in cells with acquired or primary trastuzumab resistance. These results support GDF15-mediated activation of TGF beta receptor-Src-HER2 signaling crosstalk as a novel mechanism of trastuzumab resistance.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
P38 MAPK contributes to resistance and invasiveness of HER2- overexpressing breast cancer.Curr Med Chem. 2014;21(4):501-10. doi: 10.2174/0929867320666131119155023. Curr Med Chem. 2014. PMID: 24251561 Free PMC article.
-
Transforming growth factor beta engages TACE and ErbB3 to activate phosphatidylinositol-3 kinase/Akt in ErbB2-overexpressing breast cancer and desensitizes cells to trastuzumab.Mol Cell Biol. 2008 Sep;28(18):5605-20. doi: 10.1128/MCB.00787-08. Epub 2008 Jul 14. Mol Cell Biol. 2008. PMID: 18625725 Free PMC article.
-
Pharmacologic inhibition of mTOR improves lapatinib sensitivity in HER2-overexpressing breast cancer cells with primary trastuzumab resistance.Anticancer Agents Med Chem. 2012 Feb;12(2):151-62. doi: 10.2174/187152012799015002. Anticancer Agents Med Chem. 2012. PMID: 22043997 Free PMC article.
-
Novel targeted therapies to overcome trastuzumab resistance in HER2-overexpressing metastatic breast cancer.Curr Drug Targets. 2013 Jul;14(8):889-98. doi: 10.2174/13894501113149990161. Curr Drug Targets. 2013. PMID: 23531110 Review.
-
Pharmacological strategies to overcome HER2 cross-talk and Trastuzumab resistance.Curr Med Chem. 2012;19(7):1065-75. doi: 10.2174/092986712799320691. Curr Med Chem. 2012. PMID: 22229414 Free PMC article. Review.
Cited by
-
Targeting Src family kinases in anti-cancer therapies: turning promise into triumph.Trends Pharmacol Sci. 2012 Mar;33(3):122-8. doi: 10.1016/j.tips.2011.11.002. Epub 2011 Dec 9. Trends Pharmacol Sci. 2012. PMID: 22153719 Free PMC article. Review.
-
TGF-β Signaling and Resistance to Cancer Therapy.Front Cell Dev Biol. 2021 Nov 30;9:786728. doi: 10.3389/fcell.2021.786728. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34917620 Free PMC article. Review.
-
mRNA profiling reveals determinants of trastuzumab efficiency in HER2-positive breast cancer.PLoS One. 2015 Feb 24;10(2):e0117818. doi: 10.1371/journal.pone.0117818. eCollection 2015. PLoS One. 2015. PMID: 25710561 Free PMC article.
-
NAG-1/GDF15 accumulates in the nucleus and modulates transcriptional regulation of the Smad pathway.Oncogene. 2016 Jan 21;35(3):377-88. doi: 10.1038/onc.2015.95. Epub 2015 Apr 20. Oncogene. 2016. PMID: 25893289 Free PMC article.
-
Serum Levels of Human MIC-1/GDF15 Vary in a Diurnal Pattern, Do Not Display a Profile Suggestive of a Satiety Factor and Are Related to BMI.PLoS One. 2015 Jul 24;10(7):e0133362. doi: 10.1371/journal.pone.0133362. eCollection 2015. PLoS One. 2015. PMID: 26207898 Free PMC article.
References
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. - PubMed
-
- Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L, et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol. 1996;14:737–44. - PubMed
-
- Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–48. - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. - PubMed
-
- Esteva FJ, Valero V, Booser D, Guerra LT, Murray JL, Pusztai L, et al. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:1800–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

