Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance
- PMID: 21803288
- PMCID: PMC3148494
- DOI: 10.1016/j.cmet.2011.06.008
Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance
Abstract
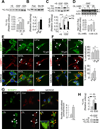
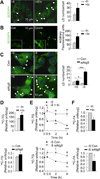
Macroautophagy is a lysosomal degradative pathway that maintains cellular homeostasis by turning over cellular components. Here we demonstrate a role for autophagy in hypothalamic agouti-related peptide (AgRP) neurons in the regulation of food intake and energy balance. We show that starvation-induced hypothalamic autophagy mobilizes neuron-intrinsic lipids to generate endogenous free fatty acids, which in turn regulate AgRP levels. The functional consequences of inhibiting autophagy are the failure to upregulate AgRP in response to starvation, and constitutive increases in hypothalamic levels of pro-opiomelanocortin and its cleavage product α-melanocyte-stimulating hormone that typically contribute to a lean phenotype. We propose a conceptual framework for considering how autophagy-regulated lipid metabolism within hypothalamic neurons may modulate neuropeptide levels to have immediate effects on food intake, as well as long-term effects on energy homeostasis. Regulation of hypothalamic autophagy could become an effective intervention in conditions such as obesity and the metabolic syndrome.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




References
-
- Belgardt BF, Bruning JC. CNS leptin and insulin action in the control of energy homeostasis. Ann N Y Acad Sci. 2010;1212:97–113. - PubMed
-
- Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224:213–232. - PubMed
-
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

