Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging
- PMID: 21803290
- PMCID: PMC3690519
- DOI: 10.1016/j.cmet.2011.05.014
Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging
Abstract
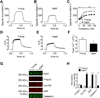
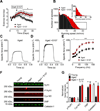
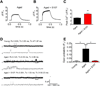
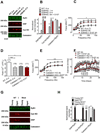
Age-related loss of muscle mass and force (sarcopenia) contributes to disability and increased mortality. Ryanodine receptor 1 (RyR1) is the skeletal muscle sarcoplasmic reticulum calcium release channel required for muscle contraction. RyR1 from aged (24 months) rodents was oxidized, cysteine-nitrosylated, and depleted of the channel-stabilizing subunit calstabin1, compared to RyR1 from younger (3-6 months) adults. This RyR1 channel complex remodeling resulted in "leaky" channels with increased open probability, leading to intracellular calcium leak in skeletal muscle. Similarly, 6-month-old mice harboring leaky RyR1-S2844D mutant channels exhibited skeletal muscle defects comparable to 24-month-old wild-type mice. Treating aged mice with S107 stabilized binding of calstabin1 to RyR1, reduced intracellular calcium leak, decreased reactive oxygen species (ROS), and enhanced tetanic Ca(2+) release, muscle-specific force, and exercise capacity. Taken together, these data indicate that leaky RyR1 contributes to age-related loss of muscle function.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest: A.R. Marks is a consultant for a start-up company, ARMGO Pharma Inc., that is targeting RyR1 to improve exercise capacity in muscle diseases
Figures



References
-
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiological Reviews. 2008;88:287–332. - PubMed
-
- Aracena-Parks P, Goonasekera SA, Gilman CP, Dirksen RT, Hidalgo C, Hamilton SL. Identification of cysteines involved in S-nitrosylation, S-glutathionylation, and oxidation to disulfides in ryanodine receptor type 1. J Biol Chem. 2006;281:40354–40368. - PubMed
-
- Aydin J, Andersson DC, Hanninen SL, Wredenberg A, Tavi P, Park CB, Larsson NG, Bruton JD, Westerblad H. Increased mitochondrial Ca2+ and decreased sarcoplasmic reticulum Ca2+ in mitochondrial myopathy. Human Molecular Genetics. 2009;18:278–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

