A mutant Ahr allele protects the embryonic kidney from hydrocarbon-induced deficits in fetal programming
- PMID: 21803694
- PMCID: PMC3261986
- DOI: 10.1289/ehp.1103692
A mutant Ahr allele protects the embryonic kidney from hydrocarbon-induced deficits in fetal programming
Abstract
Background: The use of experimental model systems has expedited the elucidation of pathogenetic mechanisms of renal developmental disease in humans and the identification of genes that orchestrate developmental programming during nephrogenesis.
Objectives: We conducted studies to evaluate the role of AHR polymorphisms in the disruption of renal developmental programming by benzo(a)pyrene (BaP).
Methods: We used metanephric cultures of C57BL/6J (C57) mice expressing the Ahr(b-1) allele and B6.D2N-Ahr(d)/J (D2N) mice expressing a mutant allele deficient in ligand binding (Ahr(d)) to investigate molecular mechanisms of renal development. Deficits in fetal programming were evaluated in the offspring of pregnant mice treated with BaP during nephrogenesis.
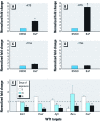
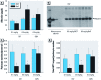
Results: Hydrocarbon challenge of metanephri from C57 mice altered Wilms' tumor suppressor gene (Wt1) mRNA splice variant ratios and reduced mRNAs of the Wt1 transcriptional targets syndecan-1 (Sdc1) paired box gene 2 (Pax2), epidermal growth factor receptor (Egfr), and retinoic acid receptor, alpha (Rarα). These changes correlated with down-regulation of effectors of differentiation [secreted frizzled-related sequence protein 1 (Sfrp1), insulin-like growth factor 1 receptor (Igf1r), wingless-related MMTV-integration site 4 (Wnt4), Lim homeobox protein 1 (Lhx1), E-cadherin]. In contrast, metanephri from D2N mice were spared hydrocarbon-induced changes in Wt1 splice variant ratios and deficits of differentiation. We observed similar patterns of dysmorphogenesis and progressive loss of renal function at postnatal weeks 7 and 52 in the offspring of pregnant C57 but not D2N mice gavaged with 0.1 or 0.5 mg/kg BaP on gestation days 10-13.
Conclusions: These findings support a functional link between AHR and WT1 in the regulation of renal morphogenesis and raise important questions about the contribution of human AHR polymorphisms to the fetal origins of adult-onset kidney disease.
Conflict of interest statement
The authors declare they have no actual or potential competing financial interests.
Figures






References
-
- Avner ED. Renal developmental diseases. Semin Nephrol. 1993;13:427–435. - PubMed
-
- Barbaux S, Niaudet P, Gubler M-C, Grünfeld J-P, Jaubert F, Kuttenn F, et al. Donor splice-site mutations in WT1 are responsible for Frasier syndrome. Nat Genet. 1997;17:467–470. - PubMed
-
- Barker DJP. The developmental origins of adult disease. J Am Coll Nutr. 2004;23(suppl 6):588S–595S. - PubMed
-
- Barnes JD, Crosby JL, Jones CM, Wright CVE, Hogan BLM. Embryonic expression of Lim-1, the mouse homolog of Xenopus XLim-1, suggests a role in lateral mesoderm differentiation and neurogenesis. Dev Biol. 1994;161:168–178. - PubMed
-
- Bertazzi PA, Pesatori AC, Zocchetti C, Latocca R. Mortality study of cancer risk among oil refinery workers. Int Arch Occup Environ Health. 1989;61(4):261–270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
