Caveolin-1 in cytokine-induced enhancement of intracellular Ca(2+) in human airway smooth muscle
- PMID: 21803870
- PMCID: PMC3191749
- DOI: 10.1152/ajplung.00019.2011
Caveolin-1 in cytokine-induced enhancement of intracellular Ca(2+) in human airway smooth muscle
Abstract
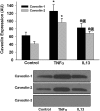
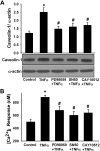
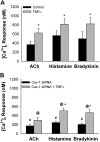
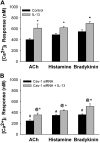
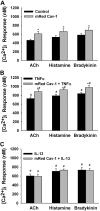
Diseases such as asthma are characterized by airway hyperresponsiveness. Enhanced airway smooth muscle (ASM) intracellular Ca(2+) ([Ca(2+)](i)) response to agonist stimulation leading to increased airway constriction has been suggested to contribute to airway hyperresponsiveness. Caveolae are flask-shaped plasma membrane invaginations that express the scaffolding protein caveolin and contain multiple proteins important in [Ca(2+)](i) signaling (e.g., agonist receptors, ion channels). We recently demonstrated that caveolae and caveolin-1 are important in [Ca(2+)](i) regulation in human ASM. Proinflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-13 modulate [Ca(2+)](i) in ASM. We hypothesized that cytokine upregulation of caveolar signaling in ASM contributes to enhanced agonist-induced [Ca(2+)](i) in inflammation. Enzymatically dissociated human ASM cells were exposed to medium (control), 20 ng/ml TNF-α, or 50 ng/ml IL-13 for 24 h. Caveolae-enriched membrane fractions displayed substantial increase in caveolin-1 and -2 expressions by TNF-α and IL-13. Transfection with caveolin-1-mRed DNA substantially accelerated and increased plasma membrane caveolin-1 expression by TNF-α and to a lesser extent by IL-13. Caveolin-1 enhancement was inhibited by nuclear factor-κB and mitogen-activated protein kinase inhibitors. In fura 2-loaded ASM cells, [Ca(2+)](i) responses to 1 μM ACh, 10 μM histamine, or 10 nM bradykinin were all exaggerated by TNF-α as well as IL-13 exposure. However, disruption of caveolae using caveolin-1 suppression via small-interfering RNA resulted in significant blunting of agonist-induced [Ca(2+)](i) responses of vehicle and TNF-α-exposed cells. These functional data were correlated to the presence of TNFR(1) receptor (but not the IL-4/IL-13 receptor) within caveolae. Overall, these results indicate that caveolin-1 plays an important role in airway inflammation by modulating the effect of specific cytokines on [Ca(2+)](i).
Figures

References
-
- Ay B, Prakash YS, Pabelick CM, Sieck GC. Store-operated Ca2+ entry in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 286: L909–L917, 2004 - PubMed
-
- Barnes PJ. Cytokine-directed therapies for asthma. J Allergy Clin Immunol 108: S72–S76, 2001 - PubMed
-
- Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev 84: 1341–1379, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

