Lachnospiraceae and Bacteroidales alternative fecal indicators reveal chronic human sewage contamination in an urban harbor
- PMID: 21803887
- PMCID: PMC3187108
- DOI: 10.1128/AEM.05480-11
Lachnospiraceae and Bacteroidales alternative fecal indicators reveal chronic human sewage contamination in an urban harbor
Abstract
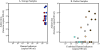
The complexity of fecal microbial communities and overlap among human and other animal sources have made it difficult to identify source-specific fecal indicator bacteria. However, the advent of next-generation sequencing technologies now provides increased sequencing power to resolve microbial community composition within and among environments. These data can be mined for information on source-specific phylotypes and/or assemblages of phylotypes (i.e., microbial signatures). We report the development of a new genetic marker for human fecal contamination identified through microbial pyrotag sequence analysis of the V6 region of the 16S rRNA gene. Sequence analysis of 37 sewage samples and comparison with database sequences revealed a human-associated phylotype within the Lachnospiraceae family, which was closely related to the genus Blautia. This phylotype, termed Lachno2, was on average the second most abundant fecal bacterial phylotype in sewage influent samples from Milwaukee, WI. We developed a quantitative PCR (qPCR) assay for Lachno2 and used it along with the qPCR-based assays for human Bacteroidales (based on the HF183 genetic marker), total Bacteroidales spp., and enterococci and the conventional Escherichia coli and enterococci plate count assays to examine the prevalence of fecal and human fecal pollution in Milwaukee's harbor. Both the conventional fecal indicators and the human-associated indicators revealed chronic fecal pollution in the harbor, with significant increases following heavy rain events and combined sewer overflows. The two human-associated genetic marker abundances were tightly correlated in the harbor, a strong indication they target the same source (i.e., human sewage). Human adenoviruses were routinely detected under all conditions in the harbor, and the probability of their occurrence increased by 154% for every 10-fold increase in the human indicator concentration. Both Lachno2 and human Bacteroidales increased specificity to detect sewage compared to general indicators, and the relationship to a human pathogen group suggests that the use of these alternative indicators will improve assessments for human health risks in urban waters.
Figures
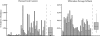


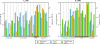
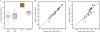
References
-
- Ahn J. H., et al. 2005. Coastal water quality impact of stormwater runoff from an urban watershed in Southern California. Environ. Sci. Technol. 39:5940–5953 - PubMed
-
- Andersson A. F., Riemann L., Bertilsson S. 2010. Pyrosequencing reveals contrasting seasonal dynamics of taxa within Baltic Sea bacterioplankton communities. ISME J. 4:171–181 - PubMed
-
- Arnone R. D., Walling J. P. 2007. Waterborne pathogens in urban watersheds. J. Water Health 5:149–162 - PubMed
-
- Barnes B., Gordon D. M. 2004. Coliform dynamics and the implications for source tracking. Environ. Microbiol. 6:501–509 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

