Standards for reporting randomized controlled trials in medical informatics: a systematic review of CONSORT adherence in RCTs on clinical decision support
- PMID: 21803926
- PMCID: PMC3240766
- DOI: 10.1136/amiajnl-2011-000411
Standards for reporting randomized controlled trials in medical informatics: a systematic review of CONSORT adherence in RCTs on clinical decision support
Abstract
Introduction: The Consolidated Standards for Reporting Trials (CONSORT) were published to standardize reporting and improve the quality of clinical trials. The objective of this study is to assess CONSORT adherence in randomized clinical trials (RCT) of disease specific clinical decision support (CDS).
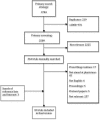
Methods: A systematic search was conducted of the Medline, EMBASE, and Cochrane databases. RCTs on CDS were assessed against CONSORT guidelines and the Jadad score.
Result: 32 of 3784 papers identified in the primary search were included in the final review. 181 702 patients and 7315 physicians participated in the selected trials. Most trials were performed in primary care (22), including 897 general practitioner offices. RCTs assessing CDS for asthma (4), diabetes (4), and hyperlipidemia (3) were the most common. Thirteen CDS systems (40%) were implemented in electronic medical records, and 14 (43%) provided automatic alerts. CONSORT and Jadad scores were generally low; the mean CONSORT score was 30.75 (95% CI 27.0 to 34.5), median score 32, range 21-38. Fourteen trials (43%) did not clearly define the study objective, and 11 studies (34%) did not include a sample size calculation. Outcome measures were adequately identified and defined in 23 (71%) trials; adverse events or side effects were not reported in 20 trials (62%). Thirteen trials (40%) were of superior quality according to the Jadad score (≥3 points). Six trials (18%) reported on long-term implementation of CDS.
Conclusion: The overall quality of reporting RCTs was low. There is a need to develop standards for reporting RCTs in medical informatics.
Conflict of interest statement
Figures
Similar articles
-
Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals.Cochrane Database Syst Rev. 2012 Nov 14;11(11):MR000030. doi: 10.1002/14651858.MR000030.pub2. Cochrane Database Syst Rev. 2012. PMID: 23152285 Free PMC article.
-
Quality of reporting of modern randomized controlled trials in medical oncology: a systematic review.J Natl Cancer Inst. 2012 Jul 3;104(13):982-9. doi: 10.1093/jnci/djs259. Epub 2012 Jul 3. J Natl Cancer Inst. 2012. PMID: 22761273
-
The reporting quality of randomised controlled trials in surgery: a systematic review.Int J Surg. 2007 Dec;5(6):413-22. doi: 10.1016/j.ijsu.2007.06.002. Epub 2007 Oct 29. Int J Surg. 2007. PMID: 18029237
-
Reporting quality and evidence support in randomized controlled trials of herbal medicine formulas for vestibular migraine.Phytomedicine. 2025 Jul 25;143:156864. doi: 10.1016/j.phymed.2025.156864. Epub 2025 May 16. Phytomedicine. 2025. PMID: 40449448
-
Endorsement for improving the quality of reports on randomized controlled trials of traditional medicine journals in Korea: a systematic review.Trials. 2014 Nov 5;15:429. doi: 10.1186/1745-6215-15-429. Trials. 2014. PMID: 25373427 Free PMC article.
Cited by
-
The quality of reports of randomized clinical trials on traditional Chinese medicine treatments: a systematic review of articles indexed in the China National Knowledge Infrastructure database from 2005 to 2012.BMC Complement Altern Med. 2014 Sep 26;14:362. doi: 10.1186/1472-6882-14-362. BMC Complement Altern Med. 2014. PMID: 25256890 Free PMC article.
-
Side-to-side versus end-to-side ileocolic anastomosis in right-sided colectomies: A cohort control study.J Minim Access Surg. 2022 Jul-Sep;18(3):408-414. doi: 10.4103/jmas.jmas_161_21. J Minim Access Surg. 2022. PMID: 35046183 Free PMC article.
-
Shifting Trends in Prostate Treatment: A Systematic Review Comparing Transurethral Resection of the Prostate and Holmium Laser Enucleation of the Prostate.Cureus. 2023 Sep 29;15(9):e46173. doi: 10.7759/cureus.46173. eCollection 2023 Sep. Cureus. 2023. PMID: 37905244 Free PMC article. Review.
-
Changes in screening, diagnosis, management, and outcomes of gestational diabetes during the COVID-19 pandemic: A systematic review.Heliyon. 2024 May 25;10(11):e31943. doi: 10.1016/j.heliyon.2024.e31943. eCollection 2024 Jun 15. Heliyon. 2024. PMID: 38845870 Free PMC article.
-
Electronic symptom reporting between patient and provider for improved health care service quality: a systematic review of randomized controlled trials. part 2: methodological quality and effects.J Med Internet Res. 2012 Oct 3;14(5):e126. doi: 10.2196/jmir.2216. J Med Internet Res. 2012. PMID: 23032363 Free PMC article.
References
-
- Moher D, Jones A, Lepage L. Use of the CONSORT statement and quality of reports of randomized trials: a comparative before-and-after evaluation. JAMA 2001;285:1992–5 - PubMed
-
- Kassell NF, Dumont AS. Randomized controlled trials in surgery: comic opera no more? J Thorac Cardiovasc Surg 2006;132:243–4 - PubMed
-
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12 - PubMed
-
- Consort 2010. Lancet 2010;375:1136 - PubMed
-
- Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA 2001;285:1987–91 - PubMed